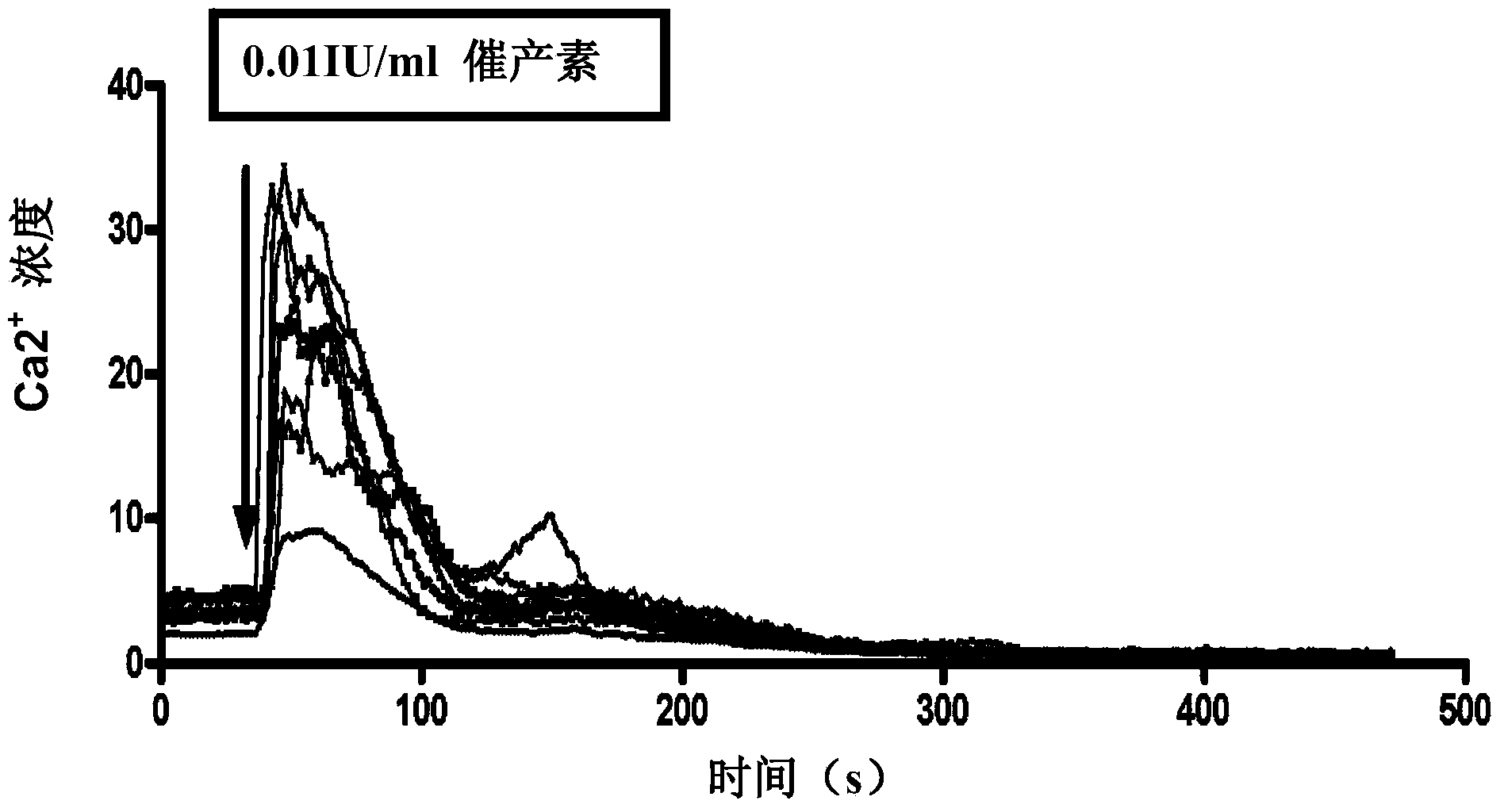
Treatment of postpartum haemorrhage with chemically modified heparin or heparan sulphate and a uterotonic agent
A technology of heparan sulfate and chemical modification, which can be used in blood diseases, active ingredients of heterocyclic compounds, medical preparations containing active ingredients, etc., and can solve problems such as no prompts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Oxidation of nonsulfated glucuronic and iduronic acids (residues), AT-binding pentasaccharides and anticoagulation active deletion
[0070] About 3000 grams of heparin was dissolved in purified water to obtain a 10-20% w / v solution. The pH of the solution was adjusted to 4.5-5.5. Then, sodium metaperiodate (NaIO 4 ) into the production solution; the amount of periodate is 15-25% of the heparin weight. The pH was again adjusted to 4.5-5.5. Protect from light for reaction. The process solution was allowed to react for 18-24 hours under continuous stirring, maintaining a temperature of 13-17°C, with the temperature dropped to 5°C during the last two hours.
[0071] Termination of oxidation reactions and removal of iodine-containing compounds
[0072] Ethanol (95-99.5%) was added to the reaction mixture over 0.5-1 hour at a temperature of 5-25°C with gentle stirring. The volume of ethanol added is in the range of 1-2 volumes of ethanol per volume of process solu...
Embodiment 2
[0080] Oxidation of glucuronic acid and iduronic acid (residues), deletion of anticoagulant activity
[0081] About 3000 grams of heparin was dissolved in purified water to obtain a 10-20% w / v solution. The pH of the solution was adjusted to 4.5-5.5. Then, sodium metaperiodate (NaIO 4 ) into the production solution; the amount of periodate is 15-25% of the heparin weight. The pH was again adjusted to 4.5-5.5. Protect from light for reaction. The process solution was allowed to react for 22-26 hours under continuous stirring, maintaining a temperature of 13-17°C, with the temperature dropped to 5°C during the last two hours. Measure and record the pH at the end of the reaction period.
[0082] Termination of oxidation reactions and removal of iodine-containing compounds
[0083] Ethanol (95-99.5%) was added to the reaction mixture over 0.5-1 hour at a temperature of 5-25°C with gentle stirring. The volume of ethanol added is in the range of 1-2 volumes of ethanol per...
Embodiment 3
[0090] Oxidation of glucuronic acid and iduronic acid (residues), deletion of anticoagulant activity
[0091] About 3000 grams of heparin was dissolved in purified water to obtain a 10-20% w / v solution. The pH of the solution was adjusted to 4.5-5.5. Then, sodium metaperiodate (NaIO 4 ) into the production solution; the amount of periodate is 15-25% of the heparin weight. The pH was again adjusted to 4.5-5.5. Protect from light for reaction. The process solution was allowed to react for 18-24 hours under continuous stirring, maintaining a temperature of 13-17°C, with the temperature dropped to 5°C during the last two hours.
[0092] Depolymerization of polysaccharide chains by the alkaline β-elimination process
[0093] While maintaining a temperature of 5-25°C, 4M NaOH solution was added slowly until a pH of 10.5-12 was obtained. Initiate and run the reaction for 15-95 minutes. At this point, the pH of the solution was recorded and 4M HCl was added slowly until a pH ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com