Sn-Mn-Ce-La catalyst as well as preparation method and application method thereof
A technology of tin manganese cerium lanthanum and its application method, which is applied in the field of tin manganese cerium lanthanum catalyst and its preparation and application method, can solve the problems of catalyst poisoning, easy deactivation, limitation of manganese catalysts, etc., and achieve elimination of temperature and low cost of raw materials , the effect of comprehensive performance improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
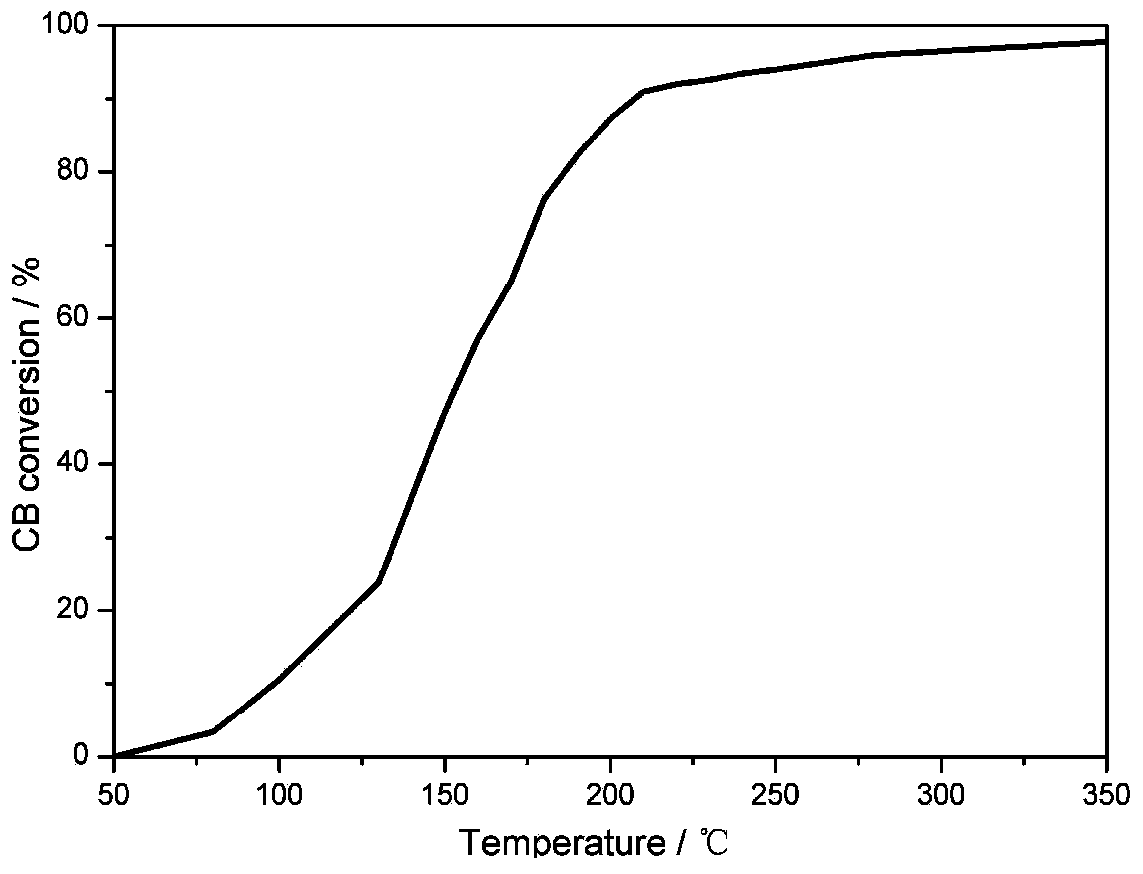
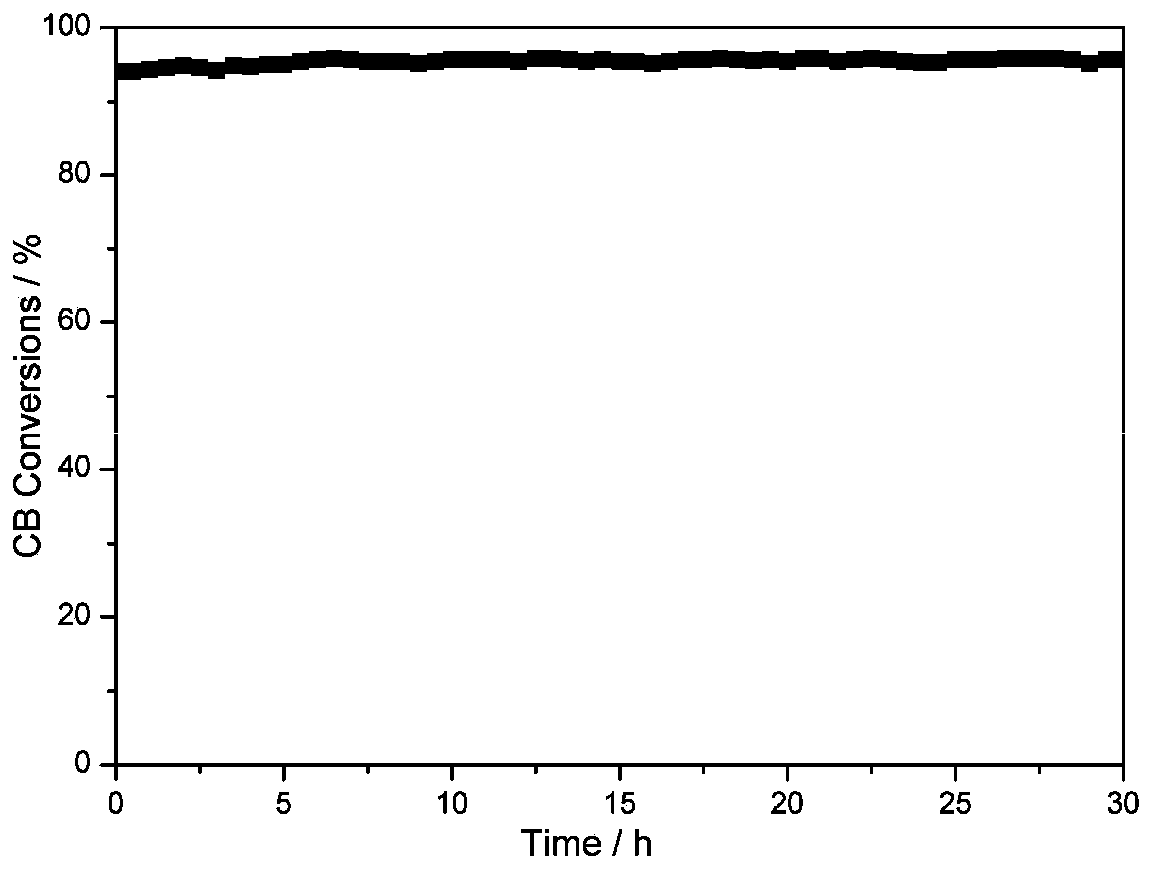
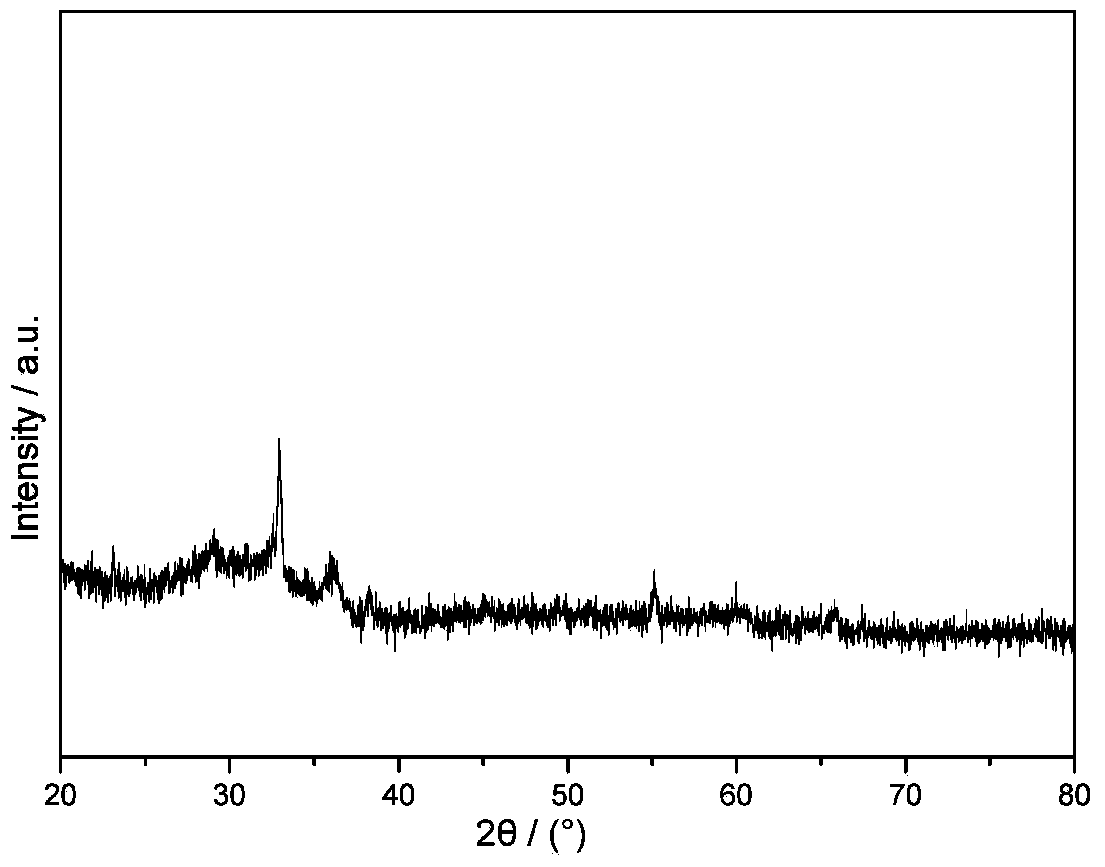
Image
Examples
Embodiment 1
[0044] The preparation method of tin manganese cerium lanthanum catalyst comprises the following steps:
[0045] 1) Weigh the metered Mn(NO 3 ) 2 , Ce(NO 3 ) 3 ·6H 2 O, La(NO 3 ) 3 ·6H 2 O, citric acid and SnCl 2 2H 2 O, according to Mn(NO 3 ) 2 , Ce(NO 3 ) 3 ·6H 2 O and La(NO 3 ) 3 ·6H 2 The ratio of the amount of O species is 12.0:1:1, Mn(NO 3 ) 2 , Ce(NO 3 ) 3 ·6H 2 O and La(NO 3 ) 3 ·6H 2 The sum of the amount of O species and SnCl 2 2H 2 The ratio of the amount of O substance is 1:0.02, and the amount of citric acid is Mn(NO 3 ) 2 , Ce(NO 3 ) 3 ·6H 2 O, La(NO 3 ) 3 ·6H 2 O and SnCl 2 2H 2 Weigh 0.25 times of the sum of the amounts of O substances;
[0046] The weighed Mn(NO 3 ) 2 , Ce(NO 3 ) 3 ·6H 2 O, La(NO 3 ) 3 ·6H 2 O, citric acid is dissolved in ultrapure water (ultrapure water quality is Mn(NO 3 ) 2 , Ce(NO 3 ) 3 ·6H 2 O, La(NO 3 ) 3 ·6H 2 O and 10 times of the mass sum of citric acid) to obtain a mixed solution, w...
Embodiment 2
[0054] The preparation method of tin manganese cerium lanthanum catalyst comprises the following steps:
[0055] 1) Weigh the metered Mn(NO 3 ) 2 , Ce(NO 3 ) 3 ·6H 2 O, La(NO 3 ) 3 ·6H 2 O, citric acid and SnCl 2 2H 2 O, according to Mn(NO 3 ) 2 , Ce(NO 3 ) 3 ·6H 2 O and La(NO 3 ) 3 ·6H 2 The ratio of the amount of O species is 11.8:1:1, Mn(NO 3 ) 2 , Ce(NO 3 ) 3 ·6H 2 O and La(NO 3 ) 3 ·6H 2 The sum of the amount of O species and SnCl 2 2H 2 The ratio of the amount of O substance is 1:0.04, and the amount of citric acid is Mn(NO 3 ) 2 , Ce(NO 3 ) 3 ·6H 2 O, La(NO 3 ) 3 ·6H 2 O and SnCl 2 2H 2 Weigh 0.3 times of the sum of the amounts of O substances;
[0056] The weighed Mn(NO 3 ) 2 , Ce(NO 3 ) 3 ·6H 2 O, La(NO 3 ) 3 ·6H 2 O, citric acid is dissolved in ultrapure water (ultrapure water quality is Mn(NO 3 ) 2 , Ce(NO 3 ) 3 ·6H 2 O, La(NO 3 ) 3 ·6H 2 O and 10 times of the mass sum of citric acid) to obtain a mixed solution, wh...
Embodiment 3
[0064] The preparation method of tin manganese cerium lanthanum catalyst comprises the following steps:
[0065] 1) Weigh the metered Mn(NO 3 ) 2 , Ce(NO 3 ) 3 ·6H 2 O, La(NO 3 ) 3 ·6H 2 O, citric acid and SnCl 2 2H 2 O, according to Mn(NO 3 )2 , Ce(NO 3 ) 3 ·6H 2 O and La(NO 3 ) 3 ·6H 2 The ratio of the amount of O species is 11.3:1:1, Mn(NO 3 ) 2 , Ce(NO 3 ) 3 ·6H 2 O and La(NO 3 ) 3 ·6H 2 The sum of the amount of O species and SnCl 2 2H 2 The ratio of the amount of O substance is 1:0.08, and the amount of citric acid is Mn(NO 3 ) 2 , Ce(NO 3 ) 3 ·6H 2 O, La(NO 3 ) 3 ·6H 2 O and SnCl 2 2H 2 Weigh 0.3 times of the sum of the amounts of O substances;
[0066] The weighed Mn(NO 3 ) 2 , Ce(NO 3 ) 3 ·6H 2 O, La(NO 3 ) 3 ·6H 2 O, citric acid is dissolved in ultrapure water (ultrapure water quality is Mn(NO 3 ) 2 , Ce(NO 3 ) 3 ·6H 2 O, La(NO 3 ) 3 ·6H 2 O and 10 times of the mass sum of citric acid) to obtain a mixed solution, whic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com