Combination of mink viral enteritis inactivated vaccine and canine distemper live vaccine
A technology for viral enteritis and inactivated vaccines, applied in antiviral agents, active ingredients of heterocyclic compounds, antibody medical ingredients, etc., can solve problems such as animal stress, canine distemper antigen failure, and the inability to realize joint use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
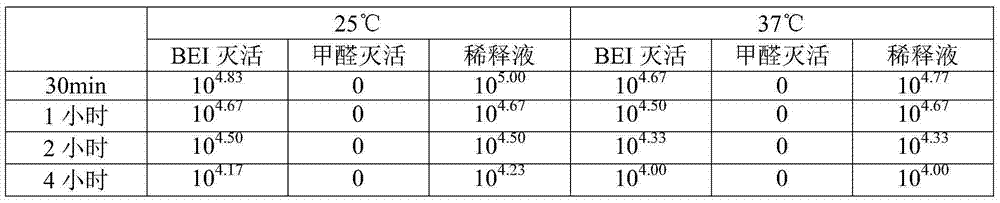
Image
Examples
preparation example Construction
[0050] (1) Preparation of seed poison for production
[0051] Canine distemper virus strain CDV-11 was inoculated on the Vero cell monolayer on the spinner bottle, and the amount of inoculation was 2% of the maintenance liquid volume. When the cytopathy reached 80%, it could be harvested, and passed on for another generation to prepare production seeds. poison.
[0052] (2) Propagation of virus liquid
[0053] The seed virus for production was used to inoculate the Vero cell monolayer on the spinner bottle, and the inoculation amount was 2% of the cell maintenance solution. Then the spinner bottle cells were cultured at 37°C with a rotation speed of 8-10r / h.
[0054] After inoculation, observe the lesions of the cells every day. When the lesions of the cells reach about 80%, they can be harvested, frozen and thawed once, and stored at -15°C. This is a semi-finished product.
[0055] (3) freeze-dried
[0056] After passing the qualified inspection of the semi-finished produ...
Embodiment 1
[0082] ——Preparation of poison seeds for production
[0083] 1. Propagation of virus seeds Take well-grown F81 cells or CRFK cells to pass passage according to conventional methods. After the cells grow into a single layer of 80%, discard the growth medium and replace it with a maintenance solution containing 1% virus seeds (containing 2% at 56 MEM of newborn bovine serum inactivated at ℃ for 30 minutes), placed at 37℃ to continue culturing, and harvested when about 80% of the cells showed typical stretch-net-like lesions, freeze-thawed once, quantitatively aliquoted, cryopreserved, and indicate the harvest Date, generation of virus species, etc.
[0084] 2. Identification of poisonous seeds The poisonous seeds that meet the above standards shall be used as the poisonous seeds for production.
[0085] 3. The subculture of poisonous seeds does not exceed 3 generations.
[0086] 4. Virus seeds are stored below -15°C, and the validity period is 24 months.
Embodiment 2
[0088] ——Manufacture of mink viral enteritis inactivated vaccine
[0089] 1. Selection of materials for making seedlings
[0090]F81 cells or CRFK cells that grow well and meet the current "Chinese Veterinary Pharmacopoeia" production and inspection cell standards are selected as materials for seedling production, and the cell generations are F6-F35 generations.
[0091] 2. Breeding of seedlings with venom
[0092] (1) Cleaning and sterilization of bioreactor and balance of microcarriers Clean the bioreactor and sterilize at 121° C. for 30 minutes. After the sterilization, put the sterilized microcarriers into the cell culture tank, put the balance medium MEM into the tank to the minimum culture volume, and balance for 24 hours. Calibration of the dissolved oxygen electrode 100% point is done during equilibration of the carrier.
[0093] (2) Cell inoculation and digestion and transfer Select vigorously growing spinner bottle cells, digest them with EDTA-trypsin dispersion, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com