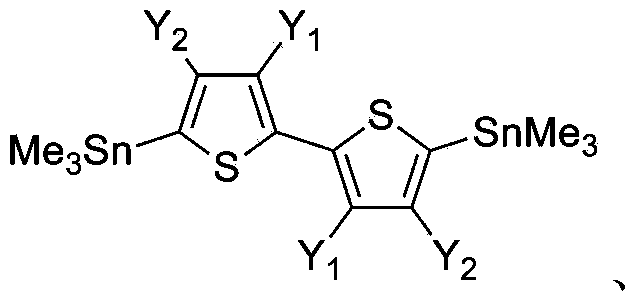
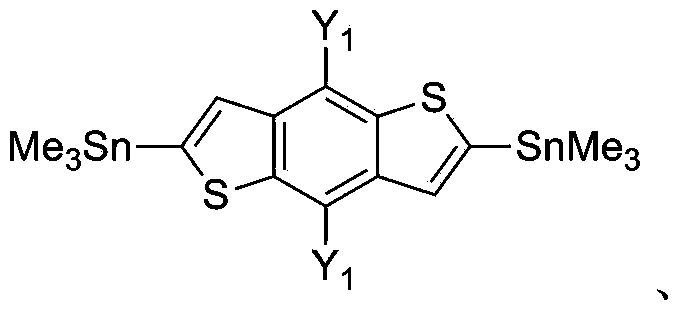
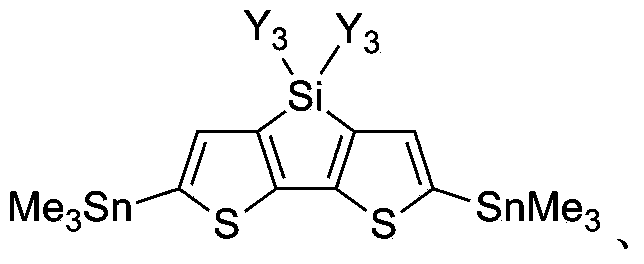
Donor material used for polymer solar battery, and polymerization monomer thereof
A technology for solar cells and polymerized monomers, which is applied to monomers with fused ring lactam structure and their preparation, and the field of donor materials for polymer solar cells. Weakness, low carrier mobility, and low energy conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0108] Synthesis Example 1: Compound TPTI is synthesized through the following reaction scheme:
[0109]
[0110] (1) Synthesis of intermediate a (2,5-dibromo-1,4-terephthaloyl chloride)
[0111] Add 2.33g of 2,5-dibromo-1,4-terephthalic acid (7.2mmol), 50mL of dichloromethane, 4mL of oxalyl chloride (45.6mmol) and 3 drops of N,N-dimethylform into a 100mL single-necked bottle Amide was stirred overnight at room temperature with the exclusion of moisture; dichloromethane and excess oxalyl chloride were spinned off, and the obtained white solid was directly used in the next reaction.
[0112] (2) Intermediate b (2,5-dibromo-N 1 ,N 4 -Bis(2-hexyldecyl)-N 1 ,N 4 -Synthesis of two (thiophene-3)-1,4-terephthalamide
[0113] Dissolve intermediate a in 20mL of dichloromethane, and add 4.7g (14.5mmol) N-(2-hexyldecyl)-3-aminothiophene dropwise under ice-water bath (for the preparation method, please refer to the literature: A New Thiophene Substituted Isoindigo Based Copolymer...
Synthetic example 2
[0126] Synthesis Example 2: Compound c2 was synthesized by the following reaction scheme:
[0127]
[0128] (1) Synthesis of intermediate a2 (2,5-dibromo-3,4-thiophene dicarboxylic acid chloride)
[0129] Add 2.38g of 2,5-dibromo-3,4-thiophenedicarboxylic acid (7.2mmol), 50mL of dichloromethane, 4mL of oxalyl chloride (45.6mmol) and 3 drops of N,N-dimethylformamide into a 100mL single-necked bottle , and stir overnight at room temperature with the exclusion of moisture; spin off dichloromethane and excess oxalyl chloride, and the white solid obtained is directly used in the next reaction.
[0130] (2) Intermediate b2 (2,5-dibromo-N 3 ,N 4 -Bis(2-hexyldecyl)-N 3,N 4 -Synthesis of two (thiophene-3)-thiophene-3,4-dicarboxamide)
[0131] Dissolve intermediate a2 in 20 mL of dichloromethane, add dropwise a dichloromethane solution containing 4.7 g (14.5 mmol) N-(2-hexyldecyl)-3-aminothiophene and 2 mL of triethylamine (20 mL ). After stirring and reacting overnight at roo...
Embodiment 3
[0135] The compound c2 obtained in Example 2 is used as a raw material for bromination to obtain compound d2, and the reaction scheme is as follows:
[0136]
[0137] Synthesis of product d2
[0138] Add c2 (288mg, 0.37mmol), 15mL chloroform and 10mL N,N-dimethylformamide to a 100mL two-necked bottle, add 136mg N-bromosuccinimide under argon protection; stir at room temperature for 24 hours and pour into 150mL Suction filtration in methanol, the crude product was purified by column chromatography using petroleum ether / dichloromethane (2:1) as the eluent to obtain 316 mg of a yellow solid (91% yield).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal decomposition temperature | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
| Short circuit current | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com