Highly pure brookite type titanium dioxide nanosheet, and preparation method and application thereof
A technology of titanium dioxide and brookite, which is applied in the direction of titanium dioxide, chemical instruments and methods, titanium oxide/hydroxide, etc., can solve the problems of harsh reaction conditions, complicated preparation process, and difficult synthesis, and achieve low alkalinity of the solution, The effect of good repeatability and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Potassium titanium oxalate was used as the initial raw material of the titanium source. At room temperature, 5.31 g of potassium titanium oxalate was dissolved in a beaker containing 60 mL of deionized water. After fully stirring, add 10 g of urea, adjust the pH to 8.5, and continue stirring until all the raw materials are dissolved. Then, the above mixed solution was put into a 100 mL reaction kettle, and placed in an oven at 200° C. to react for 12 h. After the reaction was completed, it was fully washed with ethanol and deionized water, and the obtained powder was dried in an oven at 80 °C for 12 h to obtain a white product.
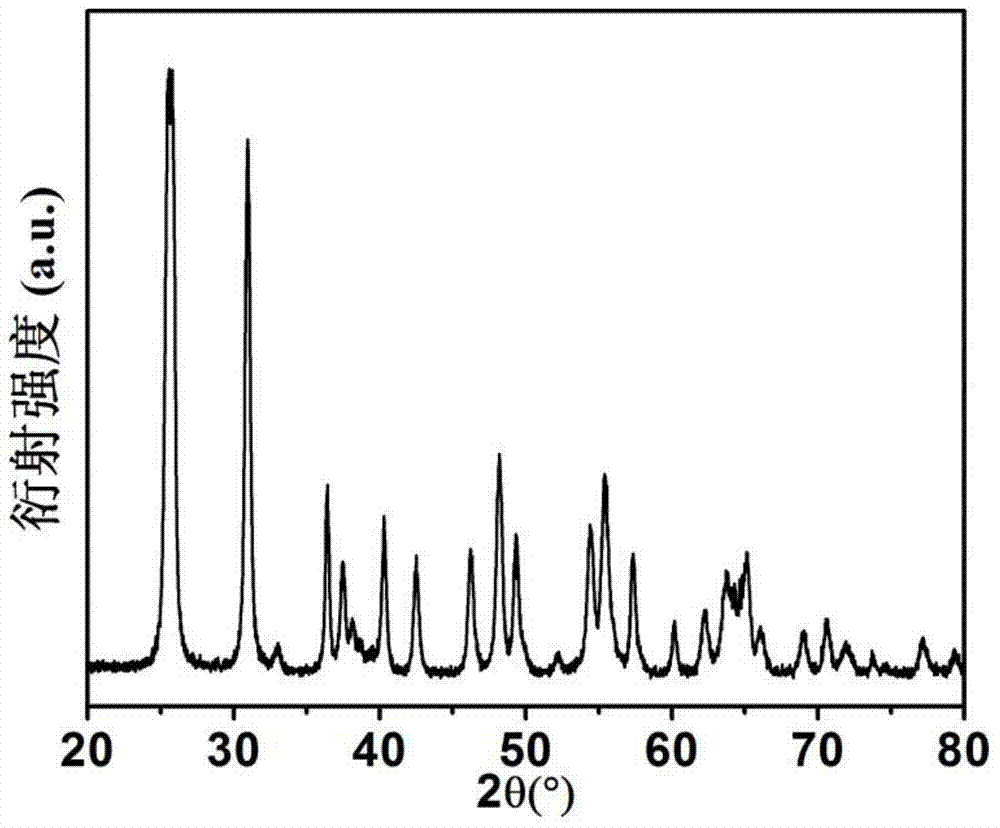
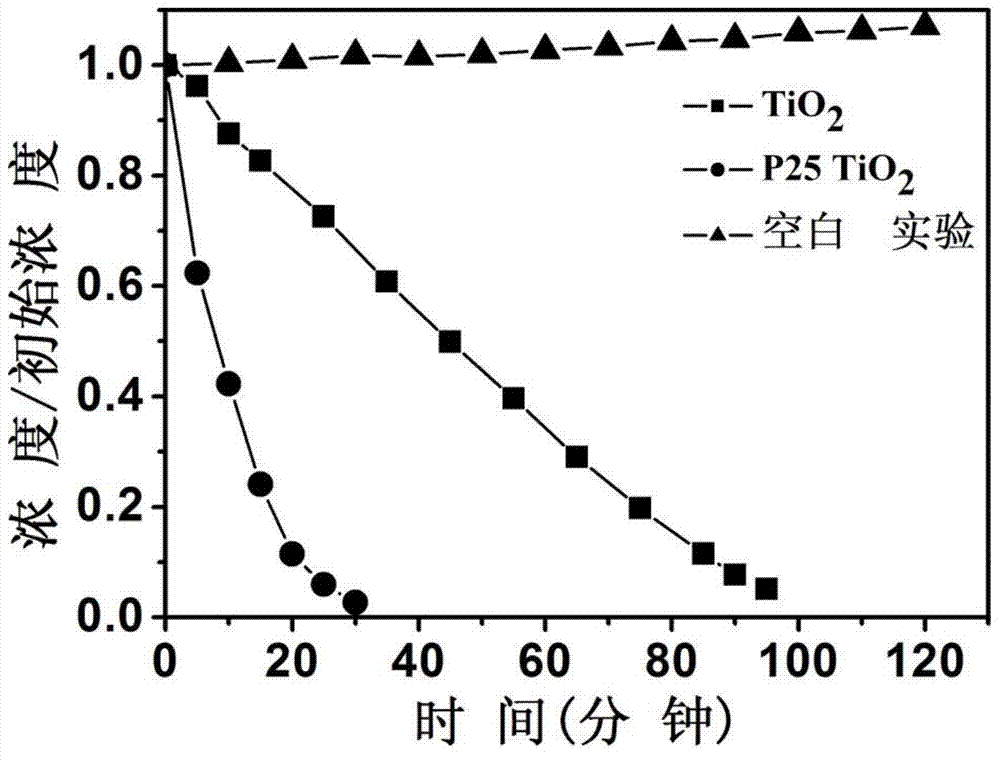
[0033] figure 1 The X-ray diffraction pattern of the titanium dioxide material prepared for this example shows that it is a pure phase brookite structure. figure 2 The transmission electron microscope photograph of the product shows that the product is flake-like particles with a particle size of 100-200 nanometers in length, 20-40 nanomete...
Embodiment 2
[0035] Potassium titanium oxalate was used as the initial raw material of the titanium source. At room temperature, 5.31 g of potassium titanium oxalate was dissolved in a beaker containing 60 mL of deionized water. After fully stirring, add 10 g of urea, adjust the pH to 9, and continue stirring until all the raw materials are dissolved. Then, the above mixed solution was put into a 100 mL reaction kettle and placed in an oven at 160° C. to react for 12 h. After the reaction was completed, it was fully washed with ethanol and deionized water, and the obtained product was dried in an oven at 80° C. for 12 h to obtain a white product.
[0036] The titania material prepared in this example is tested by X-ray diffraction pattern, which shows that the product is a pure-phase brookite structure. The transmission electron microscope photograph of the product shows that the obtained product is flake-like particles with a particle size of 100-180 nm in length, 20-35 nm in width and ...
Embodiment 3
[0038] Ammonium titanium oxalate was used as the initial raw material of the titanium source. At room temperature, 4.14 g of ammonium titanium oxalate was dissolved in a beaker with 60 mL of deionized water. After fully stirring, add 10 g of urea, adjust the pH to 9.5, and continue stirring until all the raw materials are dissolved. Then, the above mixed solution was put into a 100 mL reaction kettle, and placed in an oven at 200° C. to react for 12 h. After the reaction was completed, it was fully washed with ethanol and deionized water, and the obtained powder was dried in an oven at 80 °C for 12 h to obtain a white product.
[0039] Figure 4 The X-ray diffraction pattern of the titanium dioxide material prepared for this example shows that it is a pure phase brookite structure. Figure 5 The transmission electron microscope photograph of the product shows that the product is flake-like particles with a length of 100-200 nm, a width of 30-50 nm and a height of 15-30 nm. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com