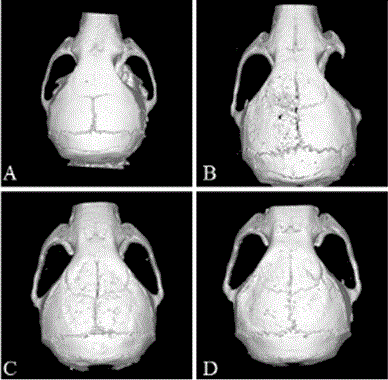
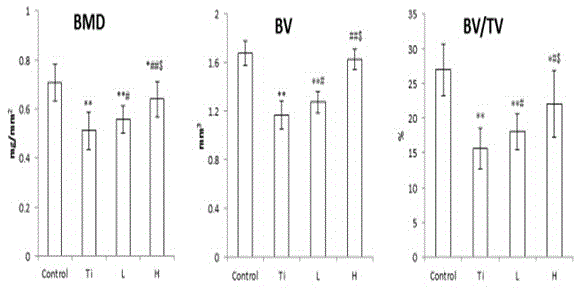
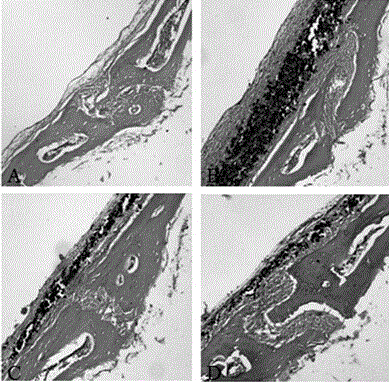
Pharmaceutical use of levodopa methyl ester hydrochloride in the treatment of periprosthetic osteolysis
A technology of levodopa methyl ester hydrochloride and periprosthetic bone, which is applied in the direction of drug combination and bone diseases, can solve the problem that there is no research on the relationship between periprosthetic osteolysis and so on, so as to inhibit the activation of osteoclasts and reduce inflammation The effect of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] 1. Materials and methods
[0025] 1. Materials
[0026] 1.1 Reagents and experimental equipment
[0027] 1.1.1 Main Drugs and Reagents
[0028]Levodopa methyl ester hydrochloride (LDME), TRAP staining kit, purchased from Sigma, the United States; paraformaldehyde, PBS, DAB chromogen, hematoxylin, eosin, absolute ethanol, distilled water, 10% chloral hydrate. Titanium particles were purchased from Johnson Mattheychemicals (catalog#00681; WardHill, Massachusetts); RANKL, OPG, TNF-α, IL-1β enzyme-linked immunosorbent assay kits were purchased from Biosource, the United States;
[0029] 1.1.2 Main instruments
[0030] Micro-CT (SkyScan1176, Belgium), paraffin slicer (Leica2135, Germany), slicer (Leica1120, Germany), paraffin embedding machine (BMJ-Ⅱ, China, Changzhou), Axiovert40C optical microscope (Zeiss, Germany), Microplate reader (Biotec, USA), a set of surgical instruments, etc.
[0031] 1.2 Experimental animals
[0032] Eighty healthy C57BL / J6 mice, male, weigh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com