Application of Tetraphenylethylene Derivatives in Detection of Methyltransferase Activity and Methyltransferase Inhibitor Concentration
A technology of methyltransferase and tetraphenylethylene, which is applied in the biological field to achieve the effects of high sensitivity, good water solubility and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] A short-chain nucleic acid modified with the quencher molecule dimethylaminoazobenzoyl at both ends, as shown in SEQ ID No: 1 (5'-GTTGGGATCGAGAG-3'), and a long-chain nucleic acid, such as SEQ ID No: 4 ( 5'-AGTGACATGATTTCCTCTCGATCCCAACCGCCGTATAGATAG-3'), mixed in an aqueous solution to obtain a quenched molecularly modified double-stranded nucleic acid (dsDNA3-2Q);
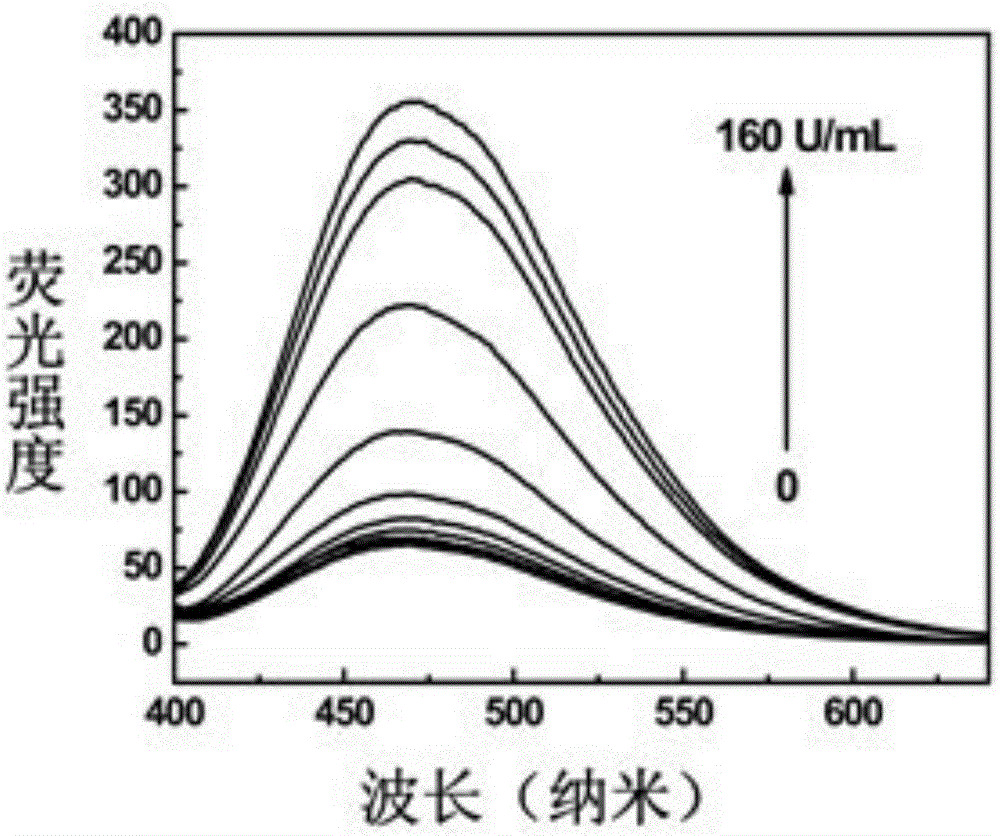
[0055] In a total reaction system of 50 μL, 2 μM dsDNA3-2Q, 1 μL enzyme reaction buffer solution [10×buffer: 200 mMTris-HAc, 500 mMKAc, 100 mMMg(Ac)2, 10 mMDTT, pH7.9], 80 μS-adenosylmethionine Acid (SAM), 400U / mL restriction endonuclease (DpnI) and different concentrations of methyltransferase Dam (concentrations were 0, 0.25, 0.5, 1, 2.5, 5, 10, 20, 40, 80 and 160U / mL), placed at 37°C for 5 hours, and then placed in a 90°C water bath for 10 minutes to inactivate Dam and DpnI, and finally cooled to room temperature to obtain a mixed solution;
[0056] The above mixed solution was mixed evenly with 50 μL ...
Embodiment 2
[0061] A short-chain nucleic acid modified with a quencher molecule dimethylaminoazobenzoyl at both ends, as shown in SEQ ID No: 2 (5'-GTTGGCCGGGAGAG-3'), and a long-chain nucleic acid, such as SEQ ID No: 5 ( 5'-AGTGACATGATTTCCTCTCCCGGCCAACCGCCGTATAGATAG-3'), mixed in an aqueous solution to obtain a double-stranded nucleic acid (dsDNA4-2Q) modified by quenching molecules;
[0062] In a total reaction system of 50 μL, mix 2 μM dsDNA4-2Q, 1 μL enzyme reaction buffer solution [10×buffer: 200 mMTris-HAc, 500 mMKAc, 100 mMMg(Ac)2, 10 mMDTT, pH7.9], 80 μS-adenosylmethionine Acid (SAM), 400U / mL restriction endonuclease (HpaII) and different concentrations of methyltransferase HpaIIMTase (concentrations were 0, 0.25, 0.5, 1, 2.5, 5, 10, 20, 40, 80 and 160U / mL), placed at 37°C for 5 hours, and then placed in a 90°C water bath for 10 minutes to inactivate HpaIIMTase and HpaII, and finally cooled to room temperature to obtain a mixed solution;
[0063] The above mixed solution was unif...
Embodiment 3
[0068] A short-chain nucleic acid with a quencher molecule dimethylaminoazobenzoyl modified at both ends, as shown in SEQ ID No: 3 (5'-GTTGGCGCGGAGAG-3'), and a long-chain nucleic acid such as SEQ ID No: 6 (5 '-AGTGACATGATTTCCTCTCCGCGCCAACCGCCGTATAGATAG-3'), mixed in an aqueous solution to obtain a double-stranded nucleic acid (dsDNA5-2Q) modified by quenching molecules;
[0069] In a total reaction system of 50 μL, 2 μM dsDNA5-2Q, 1 μL enzyme reaction buffer solution [10×buffer: 200 mMTris-HAc, 500 mMKAc, 100 mMMg(Ac)2, 10 mMDTT, pH7.9], 80 μS-adenosylmethionine acid (SAM), 400U / mL restriction endonuclease (BstUI) and different concentrations of methyltransferase M.SssI (concentrations were 0, 0.25, 0.5, 1, 2.5, 5, 10, 20, 40, and 160U / mL), placed at 37°C for 5 hours, and then placed in a 90°C water bath for 10 minutes to inactivate M.SssI and BstUI, and finally cooled to room temperature to obtain a mixed solution;
[0070]Mix the above mixed solution with 50 μL of an aqueo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com