Method for controlling high dispersion of active centers of supported metal catalyst
A technology of metal catalysts and active centers, applied in the field of highly dispersed control of supported catalyst metal centers, can solve the problem of high hydrogenolysis activity, achieve high isomerization selectivity, easy industrial application, and reduce selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
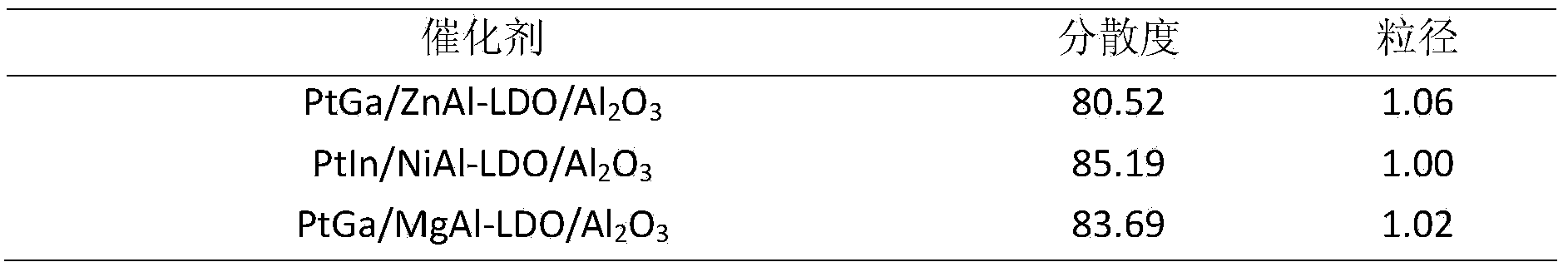
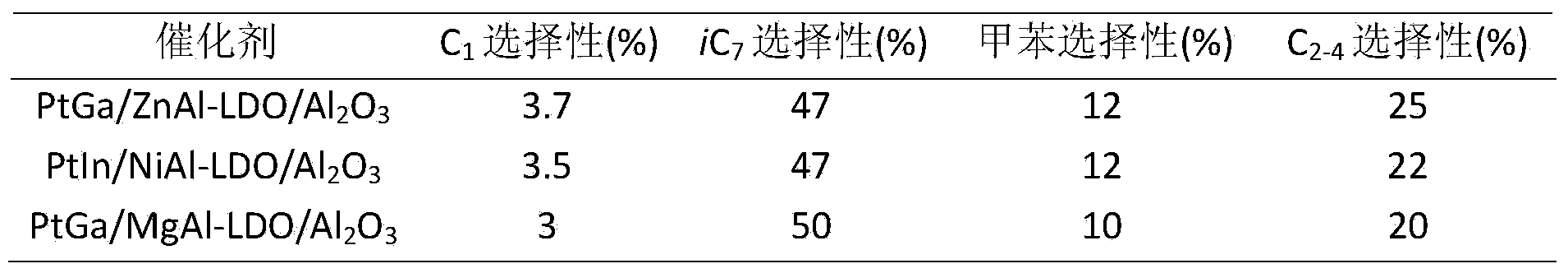
[0023]Zinc sulfate and hexamethylenetetramine were dissolved in deionized water to form solution A. Dip aluminum oxide into solution A, transfer it to a high-pressure reactor, and crystallize at 130°C for 6 hours. Wherein the molar ratio of zinc sulfate, hexamethylene tetramine and aluminum oxide is 15:80:80. After the reaction, the reaction solution was suction-filtered, washed until the eluent was neutral, and the obtained hydrotalcite-modified aluminum oxide was dried at 80° C. for 12 hours. Wherein the trivalent metal in the hydrotalcite laminate is derived from the aluminum dissolved in the reaction process of aluminum oxide.
[0024] Step B: dissolving platinum acetylacetonate and gallium acetylacetonate in a certain amount of deionized water or other solvents to form solution B, immersing the aluminum oxide modified by hydrotalcite obtained in step (1) in solution B, and standing 12h, followed by drying at 80°C for 12h. The molar ratio of platinum acetylacetonate, ga...
Embodiment 2
[0029] Nickel nitrate and ammonia water were dissolved in deionized water to form solution A. Dip aluminum oxide into solution A, transfer it to a high-pressure reactor, and crystallize at 160°C for 2 hours. Wherein the molar ratio of nickel nitrate, hexamethylene tetramine and aluminum oxide is 5:50:150. After the reaction, the reaction solution was suction-filtered, washed until the eluent was neutral, and the obtained hydrotalcite-modified aluminum oxide was dried at 80° C. for 12 hours. Wherein the trivalent metal in the hydrotalcite laminate is derived from the aluminum dissolved in the reaction process of aluminum oxide.
[0030] Step B: Sodium chloroplatinate and indium nitrate are dissolved in a certain amount of deionized water or other solvents to form a solution B, and the hydrotalcite-modified aluminum oxide obtained in step (1) is immersed in the solution B, and allowed to stand 12h, followed by drying at 80°C for 12h. The molar ratio of sodium chloroplatinate,...
Embodiment 3
[0035] Step A: Combine Magnesium Nitrate with Urea
[0036] Magnesium nitrate and urea were dissolved in deionized water to form solution A. Dip aluminum oxide into solution A, transfer it to a high-pressure reactor, and crystallize at 80°C for 12 hours. Wherein the molar ratio of magnesium nitrate, urea and aluminum oxide is 15:100:50. After the reaction, the reaction solution was suction-filtered, washed until the eluent was neutral, and the obtained hydrotalcite-modified aluminum oxide was dried at 80° C. for 12 hours. Wherein the trivalent metal in the hydrotalcite laminate is derived from the aluminum dissolved in the reaction process of aluminum oxide.
[0037] Step B: Hexaaminoplatinum nitrate and gallium nitrate are dissolved in a certain amount of deionized water or other solvents to form solution B, and the hydrotalcite-modified Al obtained in step (1) is 2 o 3 Immerse in solution B, let stand for 12h, then dry at 80°C for 12h. The molar ratio of hexaaminoplatin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com