Feed additive for anti-chicken virus disease, preparation method and application
A technology for viral diseases and feed additives, applied in antiviral agents, applications, animal feed, etc., can solve the problems of the immune effect of a single injection of yolk antibody, the effect of drugs on the safety of chicken products, and the inability to meet actual needs, so as to prevent chicken viruses. Sexual diseases, improve immunity and disease resistance, strengthen the effect of immune regulation function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
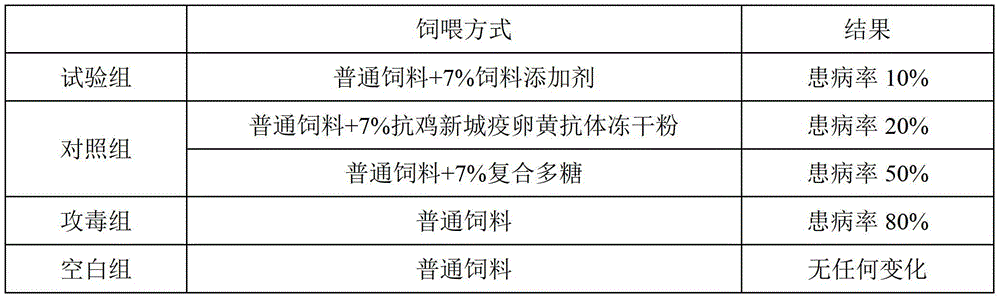
[0036] The anti-Newcastle disease yolk antibody freeze-dried powder of this embodiment includes the following components in parts by weight: 93 parts of anti-Newcastle disease yolk antibody, 0.01 part of thimerosal, 0.3 part of formaldehyde, 0.5 part of vitamin C, 1 part of polyvinylpyrrolidone, and 1 part of glycine 1 part, 1 part of sorbitol, 2 parts of glucose.
[0037] The anti-Newcastle disease feed additive of this embodiment includes the following components in parts by weight: 4 parts of anti-Newcastle disease egg yolk antibody freeze-dried powder, 5 parts of astragalus polysaccharide, 10 parts of angelica polysaccharide, 20 parts of wolfberry polysaccharide, and 30 parts of licorice polysaccharide.
[0038] The preparation method of the anti-Newcastle disease feed additive of the present embodiment may further comprise the steps:
[0039] 1) Prepare the vaccine by isolating the virus strain: Propagate and inactivate the standard Newcastle disease strain Clone30 to pre...
Embodiment 2
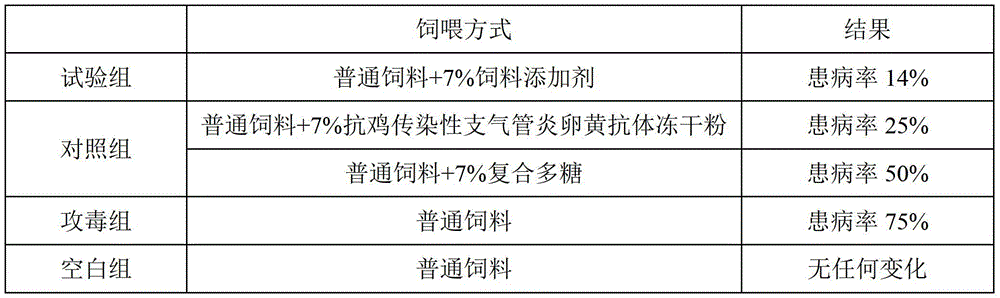
[0049] The anti-chicken infectious bronchitis egg yolk antibody freeze-dried powder of the present embodiment comprises the following components in parts by weight: 94 parts of anti-chicken infectious bronchitis egg yolk antibody, 0.01 part of thimerosal, 0.3 part of formaldehyde, 0.5 part of vitamin C, 0.5 parts of polyvinylpyrrolidone, 0.5 parts of glycine, 1 part of sorbitol, and 1.5 parts of glucose.
[0050] The anti-chicken infectious bronchitis feed additive of the present embodiment includes the following components in parts by weight: 4 parts of anti-chicken infectious bronchitis egg yolk antibody freeze-dried powder, 5 parts of astragalus polysaccharide, 10 parts of angelica polysaccharide, 20 parts of wolfberry polysaccharide 30 parts, 30 parts of licorice polysaccharide.
[0051] The preparation method of the feed additive of the anti-chicken infectious bronchitis of the present embodiment is the same as embodiment 1, and the difference is that the chicken viral di...
Embodiment 3
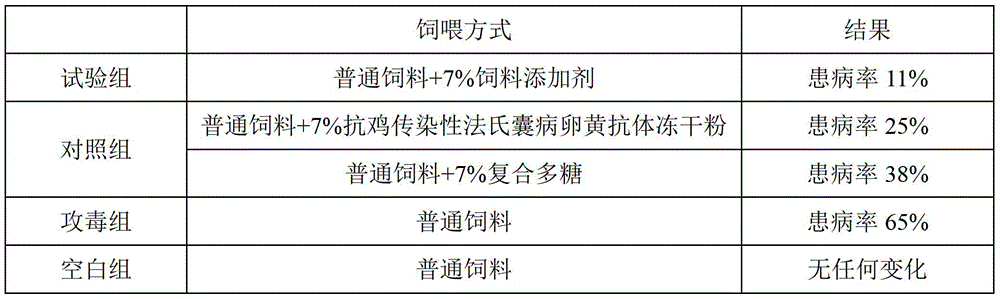
[0053] The anti-infectious bursal disease yolk antibody freeze-dried powder of the present embodiment comprises the following components in parts by weight: 95 parts of anti-infectious bursal disease yolk antibody, 0.02 part of thimerosal, 0.2 part of formaldehyde, vitamin C1 0.8 parts of polyvinylpyrrolidone, 0.8 parts of glycine, 1.5 parts of sorbitol, and 1 part of glucose.
[0054] The anti-infectious bursal disease feed additive of the present embodiment includes the following components in parts by weight: 5 parts of anti-infectious bursal disease egg yolk antibody freeze-dried powder, 8 parts of astragalus polysaccharide, and 7 parts of angelica polysaccharide , Lycium barbarum polysaccharide 15 parts, licorice polysaccharide 10 parts.
[0055] The preparation method of the feed additive of the anti-chicken infectious bursal disease of the present embodiment is the same as embodiment 1, and the difference is that the chicken viral disease virus used in the present embod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com