Cobalt carbonate-polypyrrole composite anode material used for power lithium ion battery and preparation method thereof
A negative electrode material, cobalt carbonate technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of low energy density and bulk density of negative electrodes, and achieve a simple preparation method, easy mass production, and excellent cycle stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
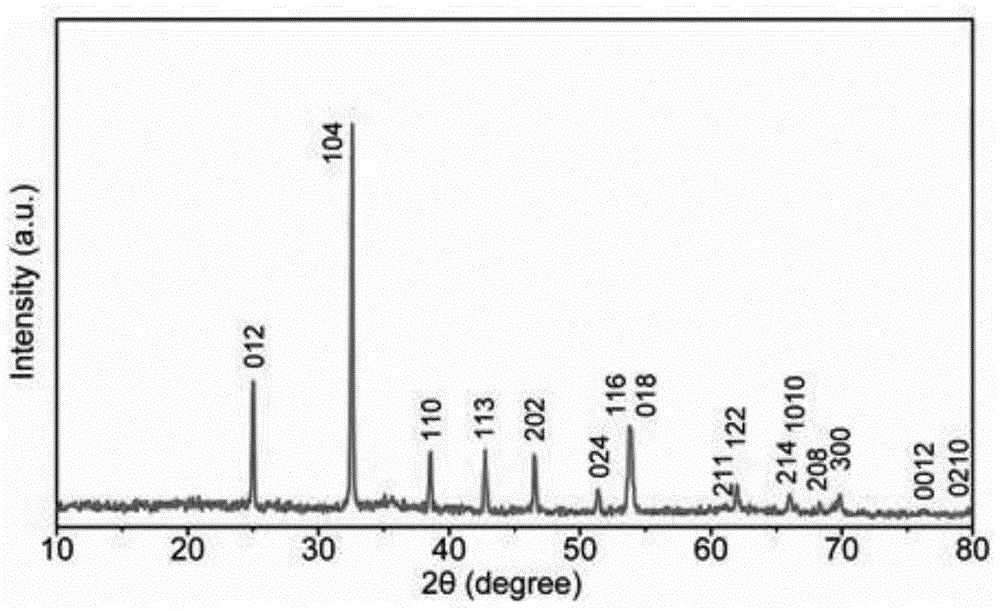
[0029] 2.8829g urea and 0.0865g CTAB were ultrasonically dissolved in 60ml deionized water to form a clear solution A; 2.8115g CoSO 4 ·7H 2 O was dissolved in 20ml of deionized water to form solution B; solution B was added dropwise to A, and after magnetic stirring for 10 minutes, 80ml of the mixed solution was transferred into a 100ml polytetrafluoroethylene liner reactor, kept at 120°C for 12h, and waited for reaction After the kettle was naturally cooled to room temperature, the hydrothermal product was successively washed with water, isopropanol and ethanol by suction filtration, and then vacuum-dried at 50°C for 12 hours to obtain rose-red CoCO 3 powder. Dissolve 0.0086g of sodium lauryl sulfate in 400ml of deionized water, then add 0.1g of hydrothermally obtained CoCO 3 Powder, ultrasonicated for 5 minutes and stirred vigorously for 2 hours to fully disperse; add 0.1g of pyrrole monomer to the above suspension and stir for 1 hour; finally add 5ml of 0.06M ferric chlor...
Embodiment 2
[0031] 12.6126g of urea and 0.6306g of CTAB were ultrasonically dissolved in 60ml of deionized water to form a clear solution A; 4.7586g of CoCl 2 ·6H 2 O was dissolved in 20 ml of deionized water to form solution B. Add solution B to A drop by drop, and after magnetically stirring for 10 minutes, transfer 80ml of the mixed solution into a 100ml polytetrafluoroethylene liner reactor and keep it warm at 180°C for 6 hours. , isopropanol and ethanol, and then vacuum-dried at 50°C for 12 hours to obtain rose red CoCO 3 powder. Dissolve 0.8120g of sodium dodecylsulfonate in 400ml of deionized water, then add 1g of hydrothermally obtained CoCO 3 Powder, ultrasonicated for 5 minutes and then vigorously stirred for 2 hours to fully disperse; add 4g of pyrrole monomer to the above suspension and stir for 1 hour; finally add 10ml of 5.96M ferric chloride solution dropwise to initiate polymerization, and continue stirring at room temperature for 2 hours . The black cobalt carbonate-...
Embodiment 3
[0033] 7.2.72g of urea and 0.2883g of CTAB were ultrasonically dissolved in 60ml of deionized water to form a clear solution A; 2.6553g of Co(CH 3 COO) 2 4H 2 O was dissolved in 20 ml of deionized water to form solution B. Add solution B to A drop by drop, and after magnetically stirring for 10 minutes, transfer 80ml of the mixed solution into a reaction kettle with a 100ml polytetrafluoroethylene liner and keep it warm at 200°C for 3 hours. , isopropanol and ethanol, and then vacuum-dried at 50°C for 12 hours to obtain rose red CoCO 3 powder. Dissolve 0.1818g of sodium dodecylbenzenesulfonate in 400ml of deionized water, then add 0.5g of hydrothermally obtained CoCO 3 Powder, ultrasonicated for 5 minutes and stirred vigorously for 2 hours to fully disperse; add 1.0g of pyrrole monomer to the above suspension and stir for 1 hour; finally add 6ml of 1.49M ferric chloride solution dropwise to initiate polymerization, and continue stirring at room temperature 24h. The black...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com