A method for resolution and preparation of optically pure r-2-naphthylethylamine
A technology of R-2-, naphthylethylamine, which is applied in the field of separation and preparation of optically pure R-2-naphthaleneethylamine, can solve the problems of high optical purity, difficult products, difficult to obtain, etc., and achieves good yield, The effect of high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
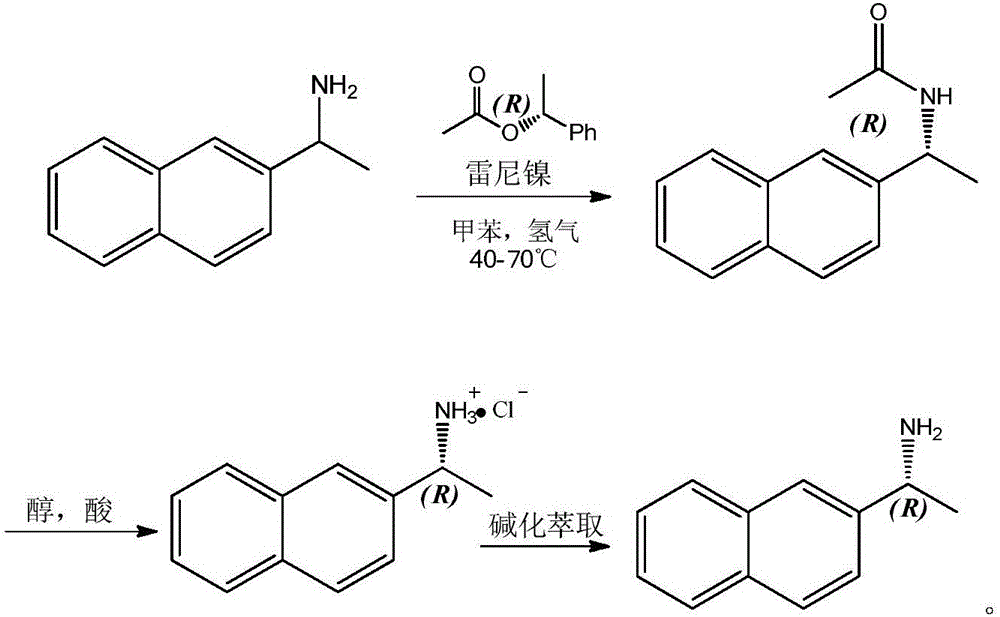
[0008] 1) Resolution and preparation of (R)-(1-(2-naphthyl)ethyl)acetamide
[0009] Add 500mL of toluene to a 1000mL autoclave as a solvent, add 85.5g of 2-naphthylethylamine, 90.2g of R-1-phenylethyl alcohol acetate, 5g of lipase novozym 435 and 8g of Raney nickel in turn, and seal the autoclave after the addition Finally, the air in the autoclave was replaced with nitrogen, and then hydrogen gas was introduced into the autoclave to a pressure of 1.0MP, stirring was started, and the temperature was raised to 60°C for reaction; after 20 hours, sampling was performed, and 2-naphthylethylamine disappeared and completely converted to (R)-(1-(2-naphthyl)ethyl)acetamide, and the ee value of the product reaches 99%; The mixed solvent was subjected to column chromatography to obtain 102.3 g of pure (R)-(1-(2-naphthyl)ethyl)acetamide with a yield of 96%.
[0010] 2) Acid hydrolysis to obtain R-2-naphthylethylamine salt
[0011] Add 53 g of (R)-(1-(2-naphthyl) ethyl) acetamide prepar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com