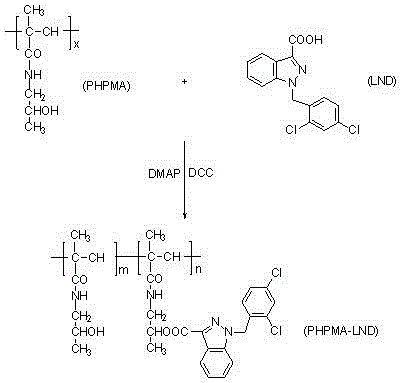Poly n-(2-hydroxypropyl) methacrylamide-lonidamine macromolecular prodrug and preparation method thereof
A technology of methacrylamide and hydroxypropyl, which is applied in the field of drug synthesis, can solve the problems of low bioavailability, low water solubility of lonidamine, and reduced therapeutic index, and achieve good biocompatibility and increase water solubility And the effect of half-life and high rate of receiving medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the synthesis of PHPMA
[0024] In a 100 mL three-neck flask, add 55 mL ethanol, 8.0 gHPMA, and 1.3 g AIBN in sequence, pass nitrogen gas under the liquid surface for 5 mins (exhaust the air), seal it, slowly raise the temperature to 55 °C and keep it for 24 h, after the reaction is complete, reduce the pressure Distill about 50 mL of ethanol, add 40 mL of ether, and stir vigorously. A large amount of white solid is formed. After suction filtration, the obtained solid is soaked in acetone for 5 h, filtered to obtain a white powder, weighing 6.8 g. Its average molecular weight is 17000 measured by viscosity method.
Embodiment 2
[0025] Embodiment 2: the synthesis of PHPMA-LND
[0026] Such as figure 1 As indicated, add 10.0 g PHPMA and 0.7 g DCC into a 100 mL round-bottomed flask, add 20 mL of freshly steamed DMF, stir and dissolve, add 1.0 g LND and 0.1 g DMAP, heat the oil bath to 50°C, and continue the reaction After 12 h, distill under reduced pressure, remove DMF, add 40 mL of acetone, a large amount of solid precipitates, filter with suction to obtain a white solid, wash with a small amount of cold isopropanol, absolute ethanol, absolute ether and acetone for several times , dried, and weighed to obtain 10.2 g of white solid, whose drug loading was determined to be 4.9% by UV.
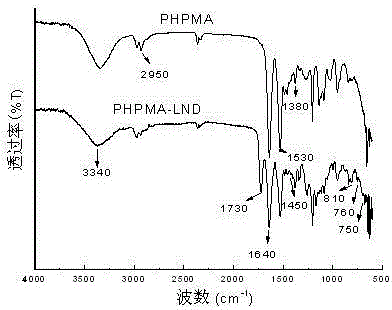
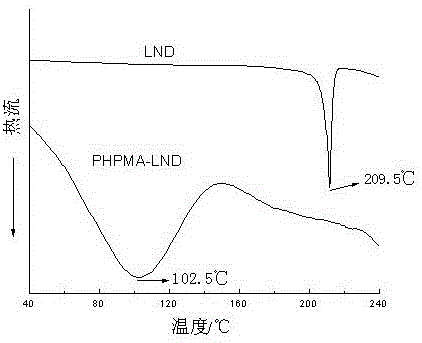
[0027] The synthesized PHPMA-LND prodrug was analyzed by IR and DSC, the results are as follows:
[0028] From figure 2 The IR spectra of PHPMA and PHPMA-LND can be seen, 3340cm -1 It is the stretching vibration absorption peak of the hydroxyl group in the polymer carrier PHPMA molecular chain, 2950 cm -1 It is th...
Embodiment 3
[0031] Embodiment 3: In vitro hydrolysis release experiment of PHPMA-LND
[0032] Put PHPMA-LND into 4cm-long dialysis bags (molecular weight cut-off 10000) and seal them respectively, and put them into 40mL of pH=1.1 (KCl-glycine / HCl), pH=7.4 (Na 2 HPO 4 / KH 2 PO 4 ), pH=8.0 (tris / CaCl 2 ), pH=10.0 (Na 2 CO 3 / NaHCO 3 ) in a buffer solution at a constant temperature of 37°C for release experiments. Sampling 5.0 mL at a certain time interval for UV testing, quantitatively determine the amount of drug released at this time interval, and at the same time replenish 5.0 mL of fresh buffer solution to the release system to maintain the solution of the release system at 40 mL. The cumulative release amount of the drug was calculated according to the following formula.
[0033] Cumulative Release (%)=100×(40.0C n + 5.0∑C(n-1)) / W 0
[0034] Where: W 0 — the mass of LND in the macromolecule prodrug to be tested (mg);
[0035] C n —Concentration (mg / mL) of LND in the relea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com