Immobilized recombinant penicillin g acylase and its application
A penicillin and acylase technology, applied in the direction of immobilization on/in organic carriers, chemical industry, sustainable manufacturing/processing, etc., can solve the problem of no immobilized carrier carrier, low immobilization efficiency and poor stability. and other problems, to achieve the effect of excellent operating stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Construction of genetically engineered bacteria Bacillus subtilis WB800 / pPZW103-PGA
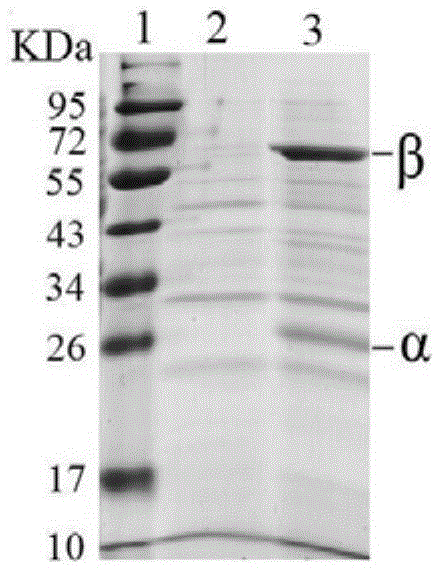
[0043] 1.1 Gene encoding recombinant penicillin G acylase
[0044] Synthesize the gene sequence of penicillin G acylase (PGA) (GenBank: AF161313.1) derived from B. megaterium CA4098 (synthesis method reference: Nucleic.Acids.Res.2005,33,W521-W525), the full length is 2046bp (shown in SEQ ID NO.2), encoding 802 amino acids, including 26 amino acid residues encoding signal peptide, 208 amino acid residues encoding α subunit, 31 amino acid residues encoding spacer peptide, and 537 encoding β subunit amino acid residues (shown in SEQ ID NO.1). The synthesized gene was connected into PES plasmid (provided by Shanghai Xuguan Biotechnology Development Co., Ltd.), and the recombinant plasmid PES-PGA was obtained, which was transformed into the cloning host Escherichia coli JM109 (provided by Beijing Tiangen Biochemical Technology Co., Ltd.), and the recombinant strain JM109 / PES was ...
Embodiment 2
[0070] Example 2 Fermentation of genetically engineered bacteria Bacillus subtilis WB800 / pPZW103-PGA
[0071] Slant culture: inoculate the Bacillus subtilis genetically engineered bacterium (Bacillus subtilis WB800 / pPZW103-PGA) constructed in Example 1 into the slant medium, culture at 37°C for 12 hours to obtain slant cells, and store at 4°C. The final concentration of the slant medium is composed of: peptone 10g / L; yeast powder 5g / L; NaCl 10g / L; agar 20g / L, pH natural, solvent is water.
[0072] Seed culture: Pick the slant strains stored at 4°C and insert them into the seed medium (50mL seed liquid medium / 250mL Erlenmeyer flask) containing kanamycin at a final concentration of 50 μg / mL, and culture overnight at 37°C and 150rpm (approx. Cultivate for 10-12 hours) to obtain seed liquid. The final concentration of the seed medium is composed of: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L, pH natural, solvent is water.
[0073] 5L fermenter cultivation: inoculate the seed s...
Embodiment 3
[0075] Example 3 Determination of Recombinant Penicillin G Acylase Activity
[0076] Get the crude enzyme liquid of the recombinant penicillin G acylase obtained by cultivating 5L fermentor in 0.2mL embodiment 2, with the N-phenylacetyl-(R, S)-o-chlorophenylglycine of 0.1mmol as substrate, with pH= 8.0 Ammonia solution was used as the reaction medium to form a 5mL reaction system, reacted in a water-bath shaker at 30°C and 180rpm for 6min, took 1mL of the reaction solution and placed it in an EP tube that had been added with 20μL of 6M NaOH aqueous solution to terminate the reaction, and placed it at 12000rpm at 4°C Centrifuge for 10 min under the conditions, take the supernatant, and detect it by HPLC.
[0077]Enzyme activity definition: the enzyme required to catalyze N-phenylacetic acid-(R,S)-2-aryl-amino acid to generate 1 μmol of (S)-2-aryl-amino acid per minute at 30°C and pH 8.0 The amount is 1 enzyme activity unit, expressed in U.
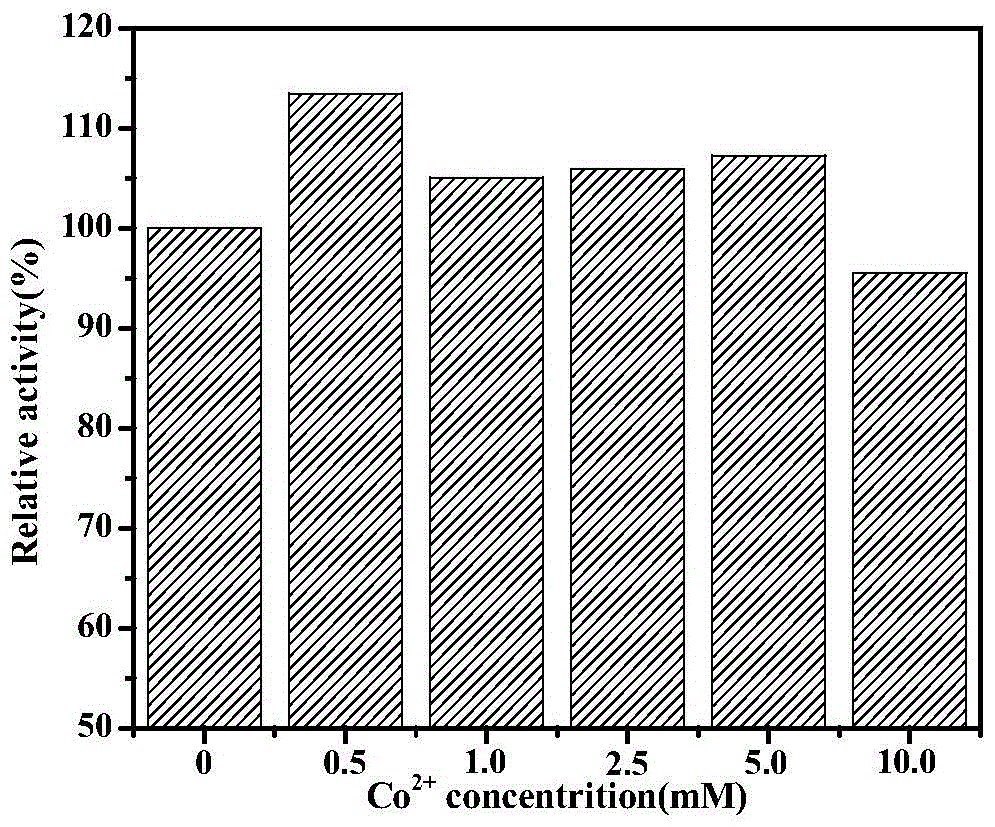
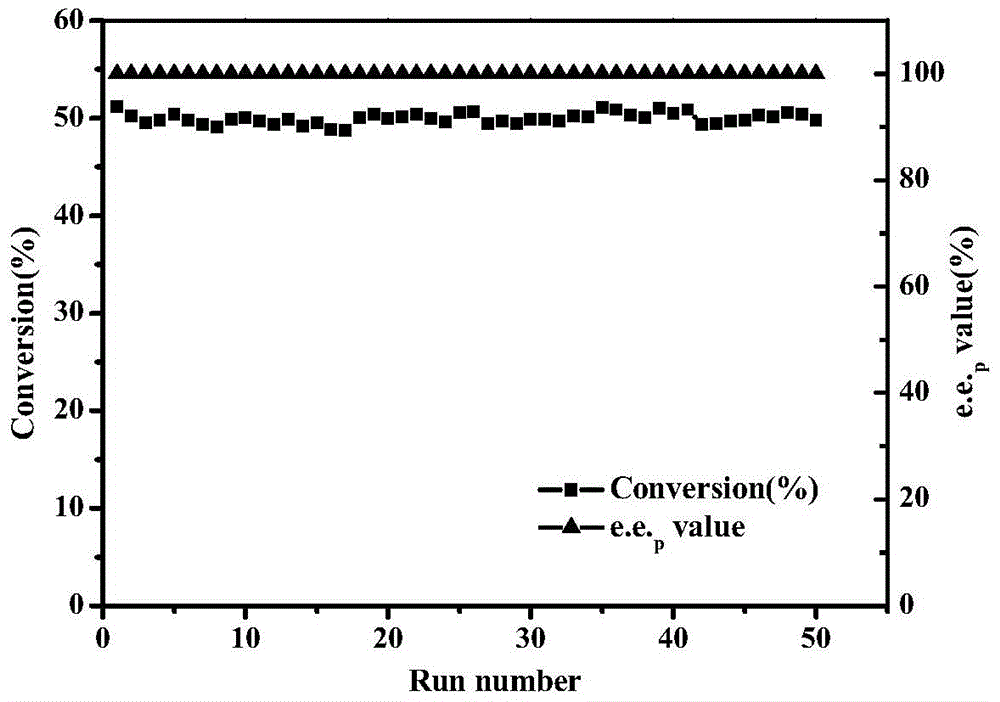
[0078] Under the above measurement...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com