Preparation method of pemetrexed disodium
A technology of pemetrexed disodium and pemetrexed acid, applied in the direction of organic chemistry, etc., can solve the problems of inability to realize industrial production, excessive N-methylation products, and decline in total yield, and achieve the purpose of inhibiting N-methylation The effect of minimizing the generation of impurities, overcoming cost increases, and improving total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
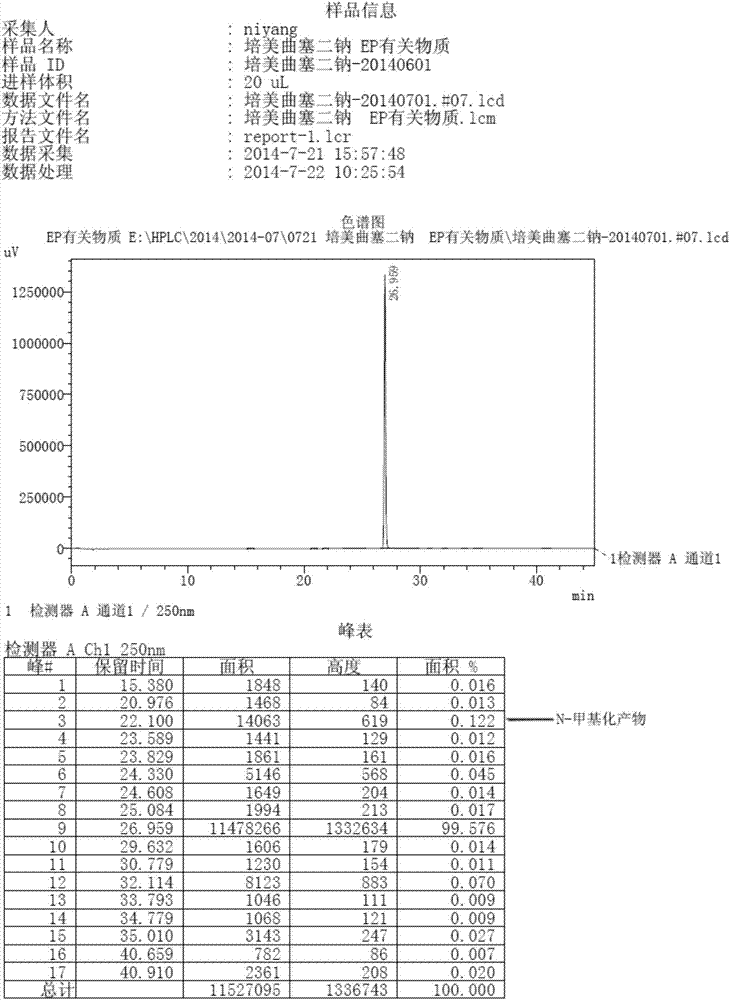
Image
Examples
Embodiment 1
[0032] The preparation method of the present embodiment pemetrexed disodium comprises the following steps:
[0033] (1) in N 2 Under protection, add 200mL tetrahydrofuran to a 500mL four-necked flask at 20°C, then continue to add 20g of N-methylmorpholine to the above-mentioned four-necked flask under stirring, continue to add 35g of 2-chloro-4 after stirring for 10min, 6-dimethoxy-1,3,5-triazine, reacted for 30min, filtered with suction, rinsed the obtained filter cake with 20mL tetrahydrofuran, filtered, dried the filter cake to obtain 46.38g 4-(4,6-dimethoxy- 1,3,5-triazin-2-yl)-4-methylmorpholine hydrochloride, spare;
[0034] (2) in N 2 Under protection, add 300mL of N,N-dimethylformamide to a 500mL three-necked flask, continue to add 50g of pemetic acid under stirring, then cool down to 0°C, and continue to add 46.38g of 4-(4,6-Dimethylformamide Methoxytriazin-2-yl)-4-methylmorpholine hydrochloride, reacted for 1h;
[0035] (3) Add 44g of L-diethyl glutamate hydrochl...
Embodiment 2
[0041] The preparation method of the present embodiment pemetrexed disodium comprises the following steps:
[0042] (1) in N 2 Under protection, add 200mL of dichloromethane to a 500mL four-necked flask at 22°C, then continue to add 45g of N-methylmorpholine to the above-mentioned four-necked flask under stirring, and continue to add 29.7g of 2-chloro -4,6-dimethoxy-1,3,5-triazine, reacted for 40min, filtered with suction, rinsed the resulting filter cake with 20mL of dichloromethane, dried the filter cake after filtering to obtain 39.4g4-(4,6- Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholine hydrochloride, spare;
[0043] (2) in N 2 Under protection, add 300mL N,N-dimethylacetamide to a 500mL three-necked flask, continue to add 50g of pemetic acid under stirring, then cool down to 1°C, and continue to add 39.4g of 4-(4,6-Dimethylacetamide Methoxy-1,3,5-triazin-2-yl)-4-methylmorpholine hydrochloride, reacted for 1.5h;
[0044] (3) Add 50 g of L-diethyl glutamate hydrochlor...
Embodiment 3
[0049] The preparation method of the present embodiment pemetrexed disodium comprises the following steps:
[0050] (1) in N 2 Under protection, add 200mL of ethyl acetate to a 500mL four-necked flask at 24°C, then continue to add 50g of N-methylmorpholine to the above-mentioned four-necked flask under stirring, and continue to add 37g of 2-chloro- 4,6-dimethoxy-1,3,5-triazine, reacted for 50min, filtered with suction, rinsed the obtained filter cake with 20mL ethyl acetate, dried the filter cake after filtering to obtain 49g of 4-(4,6-dimethyl Oxy-1,3,5-triazin-2-yl)-4-methylmorpholine hydrochloride, spare;
[0051] (2) in N 2 Under protection, add 300mL dimethyl sulfoxide to a 500mL three-necked flask, continue to add 50g of pemetic acid under stirring, then lower the temperature to 2°C, and continue to add 49g of 4-(4,6-dimethoxy-1 ,3,5-triazin-2-yl)-4-methylmorpholine hydrochloride, reacted for 1.2h;
[0052] (3) Add 65g of L-diethyl glutamate hydrochloride to the reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com