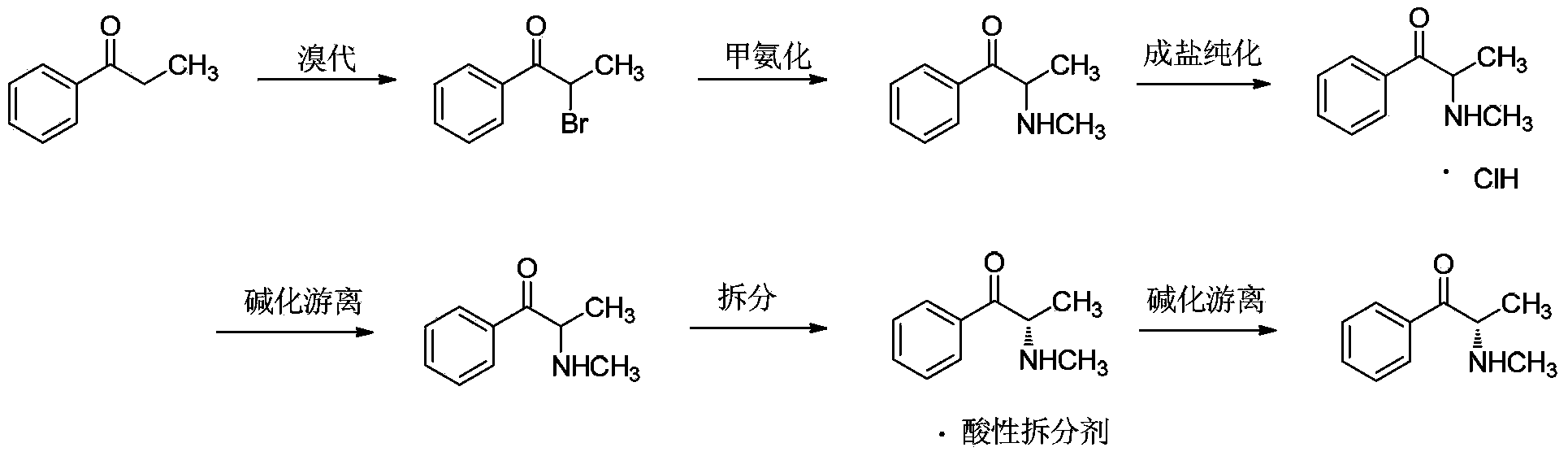
Preparation method for (S)-(-)-alpha-methylaminopropiophenone
A technology of methylaminobenzene and methylbenzoyl tartaric acid, applied in the field of medicine and chemical industry, can solve the problems that mixed solvents cannot be directly recycled and applied, and affect production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: Preparation 1 of total double salt
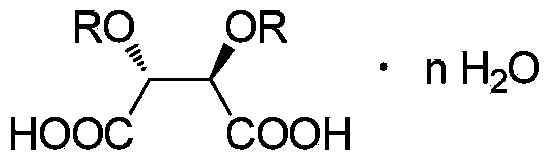
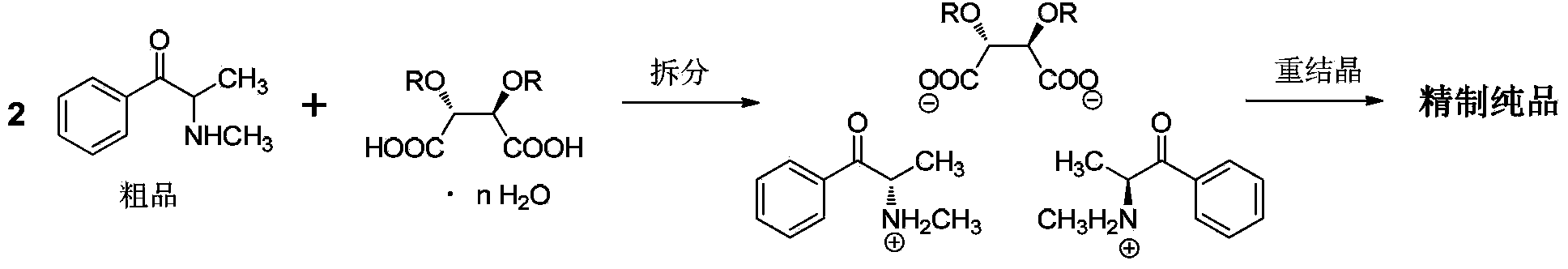
[0063] Using propiophenone as raw material, dichloromethane as reaction solvent for bromination and methylation reaction, ethyl acetate and acetic acid as resolution solvent, (2R,3R)-dibenzoyl tartaric acid as resolution agent for α -Synthesis and resolution of methylaminopropiophenone.
[0064] At room temperature, 536g of propiophenone (4mol) was dissolved in 3L of dichloromethane. Add 640g (4mol) of liquid bromine dropwise to it under mechanical stirring, control the drop rate so that the temperature of the reaction system does not exceed 30°C, and absorb the escaped hydrogen bromide with water. After the dropwise addition, continue to react for 30 minutes, wash with 1L×2 liquid separations, collect the aqueous phase, and put about 3.5L of the organic phase directly into the next step.
[0065] Equipped with a spherical condenser, mechanical stirring and heating equipment, control the internal temperature to 40°C,...
Embodiment 2
[0074] Embodiment 2: the preparation 2 of total double salt
[0075] Adopt embodiment 1 method, reaction scale is with embodiment 1. The difference is that the amount of acetic acid added in one step of resolution becomes 316mL, and the resolution system is not heated, and the method of dropping at room temperature is adopted. Stirring was continued for 14 hours after the dropwise addition was complete. All the other operations are completely consistent with Example 1.
[0076] The double salt weight that obtains is 974.6g. The total yield calculated with propiophenone as raw material was 71.1%, and the salt-forming yield of resolving agent calculated with the amount of (2R,3R)-dibenzoyl tartaric acid was 87.9%. The purity of the total double salt was 99.0%, and the %de value was 86.1%.
Embodiment 3
[0078] Embodiment 3: the preparation 3 of total double salt
[0079] Using the method of Example 1, the reaction scale is 10% of Example 1 (that is, all materials are reduced to 10%, such as raw material propiophenone reduced to 53.6g). Ethyl acetate was used as the solvent for bromination and methylation, and other operations were unchanged.
[0080] The double salt weight that obtains is 83.7g. The purity of the total double salt was 98.0%, and the %de value was 91.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com