A class of 1,3-dihydro-1-oxo-2h-isoindole compounds and their uses
A technology for oxoisoindole and compounds, which is applied in the field of a class of 1,3-dihydro-1-oxo-2H-isoindole compounds and their uses, and can solve the problems of low bioavailability, difficulty in mass production, and half-life Short and other questions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
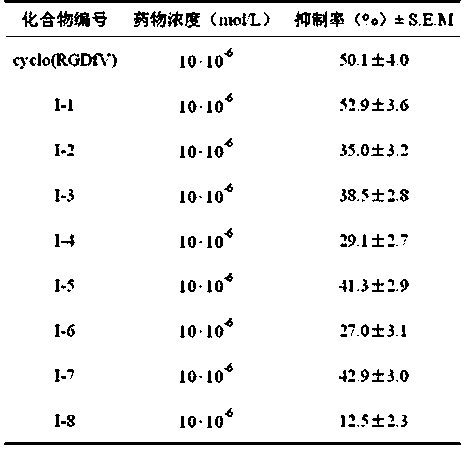
[0044] Preparation of 3-(6-(3-guanidinobenzamido)-1-oxoisoindol-2-yl)-3-phenylpropion hydrochloride (I-1) and trifluoroacetate
[0045]
[0046] Step (a): Add 10.0 mmol of 2-formyl-5-nitrobenzoic acid (4), 12.0 mmol of β-phenyl-β-alanine methyl ester and 80 ml of methanol into the reaction flask, and stir at room temperature for 2.0 h , slowly add NaBH under cooling in an ice-water bath 4 10.0 mmol, insulated and stirred for reaction (the reaction process was monitored by TLC), after the reaction was completed, the solvent was evaporated under reduced pressure, 20 ml of acetic acid was added to the residue, and the reaction was incubated at 80°C for 40 min, and 100 ml of deionized water was added to the reaction bottle, and the precipitated Solid, the filter cake was washed with aqueous sodium carbonate solution and dried to obtain 3-(6-nitro-1-oxoisoindol-2-yl)-3-phenylpropionic acid methyl ester with a yield of 90.0%, ESI- MS ( m / z , +Q): 341.0 [M+H] + ;
[0047] St...
Embodiment 2
[0052] Preparation of 3-(6-(4-guanidinobenzamido)-1-oxoisoindol-2-yl)-3-phenylpropion hydrochloride (I-2)
[0053]
[0054] The operation process is the same as in Example 1, except that the m-guanidine benzo hydrochloride in the step (c) is replaced with p-guanidine benzo hydrochloride, and the condensation agent 1-ethyl-3-(3-dimethyl Aminopropyl) carbodiimide hydrochloride is replaced by DCC to obtain 3-(6-(4-guanidinobenzamido)-1-oxoisoindol-2-yl)-3-phenylpropionic acid Hydrochloride, yield 74.0%, 1 H NMR (400MHz, DMSO-d 6 ) δ: 12.42(brs, 1H, COOH), 11.01(brs, 1H, HCl), 10.64(s, 1H, CONH), 8.26(d, 1H, J =1.6Hz, Ar-H), 8.11(d, 2H, J =8.4Hz, Ar-H), 7.98(dd, 1H, J 1 / =1.6Hz, J 2 / =8.0Hz, Ar-H), 7.97(brs, 4H, NH 2 C(NH)NH), 7.52(d, 1H, J =8.0Hz, Ar-H), 7.33(d, 2H, J =8.4Hz, Ar-H), 7.40-7.27(m, 5H, Ar-H), 5.77(t, 1H, J =8.0Hz, CH), 4.52(d, 1H, J =17.6Hz, ArCH 2 ), 4.14(d, 1H, J =17.6Hz, ArCH 2 ), 3.13-3.02(m, 2H, CH 2 COOH); ESI-MS ( m / z ): 458.15 [M-Cl] + ...
Embodiment 3
[0056] 3-[6-[3-[(4,5-Dihydro-1 H Preparation of -imidazol-2-yl)amino]benzamido]-1-oxoisoindol-2-yl]-3-phenylpropion hydrochloride (I-3)
[0057]
[0058] The operation process is the same as in Example 1, except that the m-guanidinobenzoic acid hydrochloride in the step (c) is treated with 3-[(4,5-dihydro-1 H -imidazol-2-yl) amino] benzoic acid hydrochloride instead, condensing agent 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride replaces with DCC, obtains 3-[ 6-[3-[(4,5-dihydro-1 H -Imidazol-2-yl)amino]benzamido]-1-oxoisoindol-2-yl]-3-phenylpropion hydrochloride, yield 85.0%, 1 H NMR (400MHz, DMSO-d 6 ) δ: 12.44(brs, 1H, COOH), 10.89(s, 1H, HCl), 10.78(s, 1H, CONH), 8.58(s, 2H, NHCNH), 8.25(d, 1H, J =1.2Hz, Ar-H), 7.95(dd, 1H, J 1 / =1.2Hz, J 2 =8.4Hz, Ar-H), 7.88-7.86(m, 2H, Ar-H), 7.59(t, 1H, J =8.0Hz, Ar-H), 7.53(d, 1H, J =8.0Hz, Ar-H), 7.45(d, 2H, J =8.0Hz, Ar-H), 7.41-7.28(m, 5H, Ar-H), 5.76(t, 1H, J =8.0Hz, CH), 4.50(d, 1H, J =17.6Hz, ArCH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com