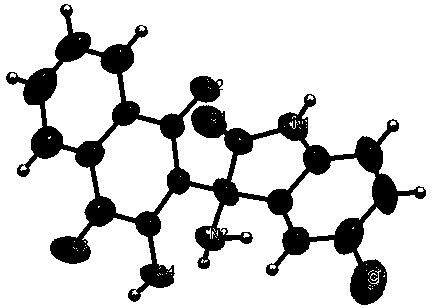
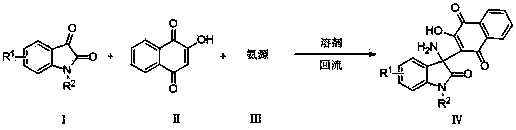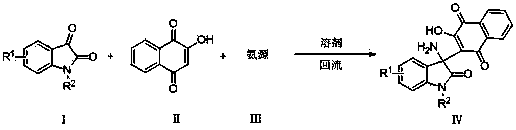2-(3-amino-2-oxoindolin-3-yl)-3-hydroxyl-1,4-naphthoquinone derivative and preparation method thereof
A technology for indole alkanes and derivatives is applied in the field of 2--3-hydroxy-1,4-naphthoquinone derivatives and their preparation, and achieves the effects of high yield, mild reaction conditions and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: (IV-1) Preparation of 2-(3-amino-2-oxoindoalkyl-3-yl)-3-hydroxyl-1,4-naphthoquinone
[0025] Add isatin (147mg, 1mmol), 2-hydroxy-1,4-naphthoquinone (174 mg, 1mmol), ammonium acetate (116 mg, 1.5mmol) into ethanol (5ml), heat to reflux, track by TLC, 2h After the reaction is over, cool, filter with suction, wash, and dry to obtain the orange solid product (IV-1) 2-(3-amino-2-oxoindolane-3-yl)-3-hydroxyl-1,4- Naphthoquinone (314 mg, 98%), m.p. 266-268°C.
[0026] 1 H NMR (500 MHz, DMSO– d 6 ) δ 10.64 (s, 1H), 9.30 (s, 3H), 7.83 (d, J = 7.5 Hz, 2H), 7.71 (t, J = 7.1 Hz, 1H), 7.59 (td, J = 7.5, 1.2 Hz, 1H), 7.42 (d, J = 7.4 Hz, 1H), 7.25 (td, J = 7.7, 1.1 Hz, 1H), 6.91 (t, J = 7.6 Hz, 1H), 6.87 (d, J = 7.8 Hz, 1H); 13 C NMR (126 MHz, DMSO– d 6 ) δ 184.0, 174.6, 142.7, 133.9, 131.0, 130.9, 129.8, 129.1, 125.4, 125.1, 124.6, 121.7, 109.6, 61.3;
[0027] ESI-MS (m / z): 321.0 [M+H] + .
[0028] Among the above-mentioned examples, the source of ...
Embodiment 2
[0029] Example 2: (IV-1) Preparation of 2-(3-amino-2-oxoindolane-3-yl)-3-hydroxyl-1,4-naphthoquinone
[0030] The same operation as in Example 1, adding glacial acetic acid (0.01ml, 0.2mmol), and refluxing for 2 h, the orange solid product (IV-1) 2-(3-amino-2-oxoindolane-3- yl)-3-hydroxy-1,4-naphthoquinone (299 mg, 93%).
Embodiment 3
[0031] Example 3: (Ⅳ-1) Preparation of 2-(3-amino-2-oxoindolane-3-yl)-3-hydroxyl-1,4-naphthoquinone
[0032] With the operation of Example 1, cesium carbonate (65 mg, 0.2 mmol) was added, and after reflux for 2 h, the orange solid product (IV-1) 2-(3-amino-2-oxoindolane-3-yl )-3-Hydroxy-1,4-naphthoquinone (250 mg, 78%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com