Carbon dioxide capturing method
A technology based on carbon dioxide and primary amines, applied in chemical instruments and methods, separation methods, through absorption, etc., can solve problems such as easy deposition and coalescence, blockage of equipment, and impact on system fluidity, so as to improve capture efficiency and decarbonization The effect of high efficiency and strong solvency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
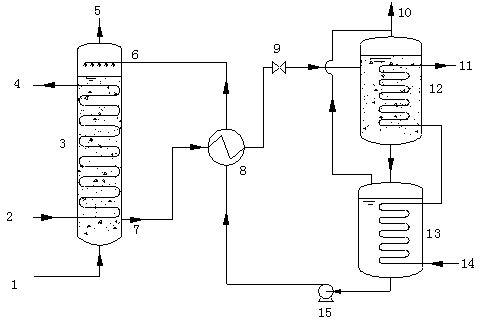
Image
Examples
Embodiment 1
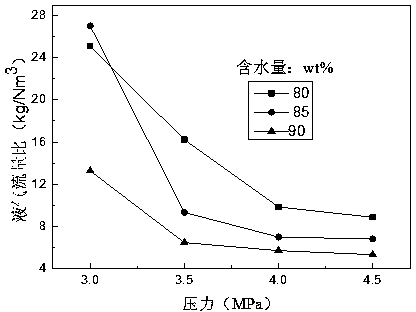
[0041] Embodiment 1 The relationship between liquid-gas flow ratio and working pressure
[0042] Set the feed gas, CO 2 The volume fraction is 95%, and set at the same decarburization efficiency, change the concentration of ionic liquid, liquid gas flow rate and working pressure in the absorption tower, and investigate the changes, the results are as follows figure 2 shown. At a certain concentration of ionic liquid, the liquid-gas flow ratio decreases with the increase of working pressure. This is due to the higher the pressure, the CO absorbed by the solution 2 more, and CO 2 Hydrates are easier to generate and capture the same CO 2 The smaller the amount of solution required. After the pressure increases to a certain value (4.5MPa), the liquid-gas flow ratio decreases very slowly. Under the same working pressure, the greater the water content of the ionic liquid aqueous solution, the smaller the liquid-gas flow ratio in the process. This is because the greater the w...
Embodiment 2
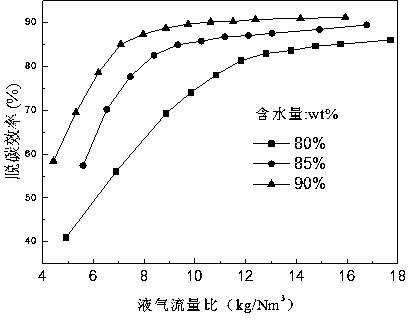
[0043] Example 2 Liquid-gas flow ratio vs. decarburization efficiency
[0044] Set the feed gas, CO 2 The volume fraction is 95%, the working pressure is 4.0 MPa, and the decarburization efficiency under different liquid-gas flow ratios is as follows: image 3 shown. The decarburization efficiency of ionic liquid solutions with different water contents tends to a certain value with the increase of liquid-gas flow ratio. When the liquid-gas flow ratio is large enough, the maximum decarburization efficiency is about 91% when the water content is 90 wt%, and the maximum decarburization efficiency is 88% and 85% when the water content is 85 wt% and 80 wt%, respectively. After testing, it was found that under different water content conditions, there is an optimal liquid-gas flow ratio (water content is 90wt%, 85wt% and 80wt%, the best liquid-gas flow ratio is 10.62 kg 溶液 / Nm 3 , 11.18 kg 溶液 / Nm 3 and 11.8 kg 溶液 / Nm 3 ), under the optimum liquid-gas flow ratio, it can ensur...
Embodiment 3
[0045] Example 3 Working pressure on decarburization efficiency
[0046] Set the feed gas, CO 2 The volume fraction is 95%, and the CO in the exhaust gas of the absorption tower is used 2 volume fraction to reflect the decarburization effect. The result is as Figure 4 As shown, the CO in the exhaust gas of the absorber 2 The volume fraction decreases almost linearly with the working pressure, indicating that high working pressure is beneficial to CO 2 capture. Higher pressure is not only beneficial for hydrates to capture CO 2 , can also enhance the CO 2 physical absorption. But the working pressure needs to be adjusted in combination with the temperature and according to the actual situation.
[0047] Depend on Figure 5 It can be seen that gas hydrates and ionic liquid solutions each capture CO 2 The relationship between the ratio of the quantity ( ) and the working pressure. Under the conditions of high water concentration and high pressure, CO hydrates are form...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com