Method for producing acetoin by efficient bioconversion of 2,3-butanediol by using Bacillus subtilis nicotinamide adenine dinucleotide (NAD)<+> regeneration system
A technology of acetoin and butanediol, applied in the fields of genetic engineering and bioengineering, can solve the problems of low accumulation concentration, difficult separation in downstream engineering, inability to obtain pure acetoin, etc., and achieves efficient production, cost reduction, and simplified separation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: Amplification of target gene and construction of recombinant Bacillus subtilis
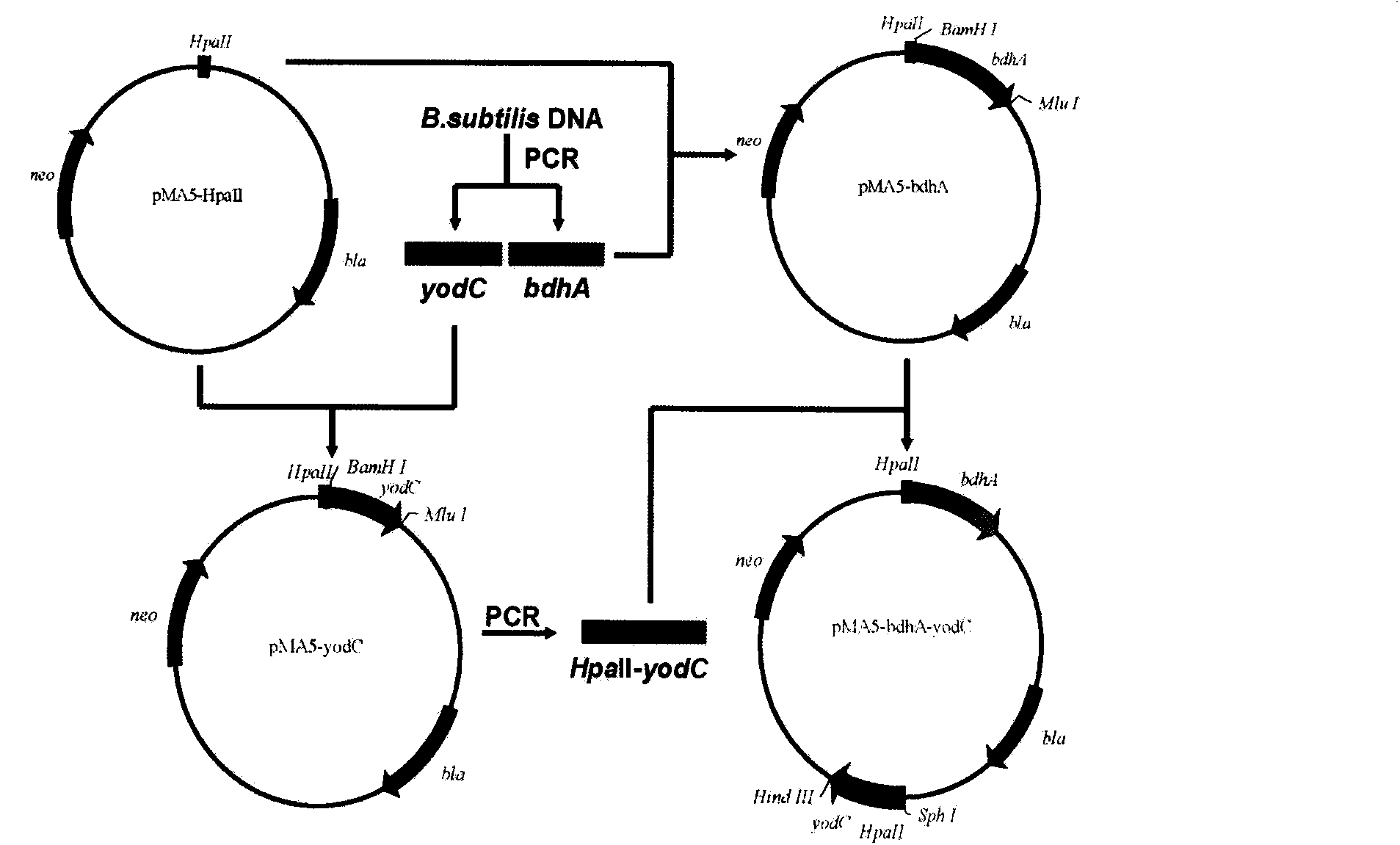
[0015] The schematic diagram of the construction of plasmid pMA5-bdhA-yodC is as follows figure 1 As shown, the specific process is as follows:
[0016] First, using the chromosomal DNA of the bacterial strain B.subtilis JNA as a template, using primers P1 and P2, a nucleotide sequence of 1041 bp is amplified by PCR to obtain a bdhA gene as shown in SEQ ID NO: 1, and the purified After the bdhA gene was digested by restriction endonucleases MluI and BamHI, it was connected with the plasmid pMA5 digested by the above two restriction endonucleases to construct the recombinant plasmid pMA5-bdhA. Plasmid construction was successful. Subsequently, using primers P3 and P4 to obtain the gene yodC whose nucleotide sequence is shown in SEQ ID NO: 2 by PCR amplification, after the purified yodC gene was digested by restriction endonucleases MluI and BamHI, the same process as describe...
Embodiment 2
[0025] Embodiment 2: Determination of recombinant strain enzyme activity
[0026] (1) Enzyme activity assay buffer system
[0027] Acetoin reductase: 0.05M acetoin, 50mM sodium phosphate buffer pH6.5, 5mM NAD + ;
[0028] 2,3-butanediol dehydrogenase: 0.1M 2,3-butanediol, 50mM sodium phosphate buffer pH8.0, 5mM NADH;
[0029] NADH oxidase: 50mM potassium phosphate buffer pH7.0, 0.3mM EDTA, 50μM FAD, 0.3mMβ-NADH;
[0030] (2) Determination of enzyme activity
[0031] The recombinant bacteria B.subtilis JNA / pMA5-bdhA, B.subtilis JNA / pMA5-yodC and B.subtilis JNA / pMA5-bdhA-yodC constructed in Example 1 were inoculated with the starting strain B.subtilis JNA in 10 mL of card-containing In the LB medium of namycin, shake culture overnight at 37°C, transfer to LB medium the next day at 4% inoculum size, culture at 37°C for 24 hours, take the fermentation broth at 4°C, centrifuge at 10000r / min for 10min, pH7 .0 sodium phosphate buffer, washed three times, suspended in pH 7.0 sodi...
Embodiment 3
[0032] Example 3: Determination of intracellular cofactor levels and whole cell viability of recombinant bacterial strains
[0033] The intracellular cofactor levels of the recombinant strains were detected using the AAT Bioquest reagent kit. Detect NADH, NAD+ concentration and NADH / NAD at Ex / Em=540 / 590nm by fluorescent microplate reader + Proportion.
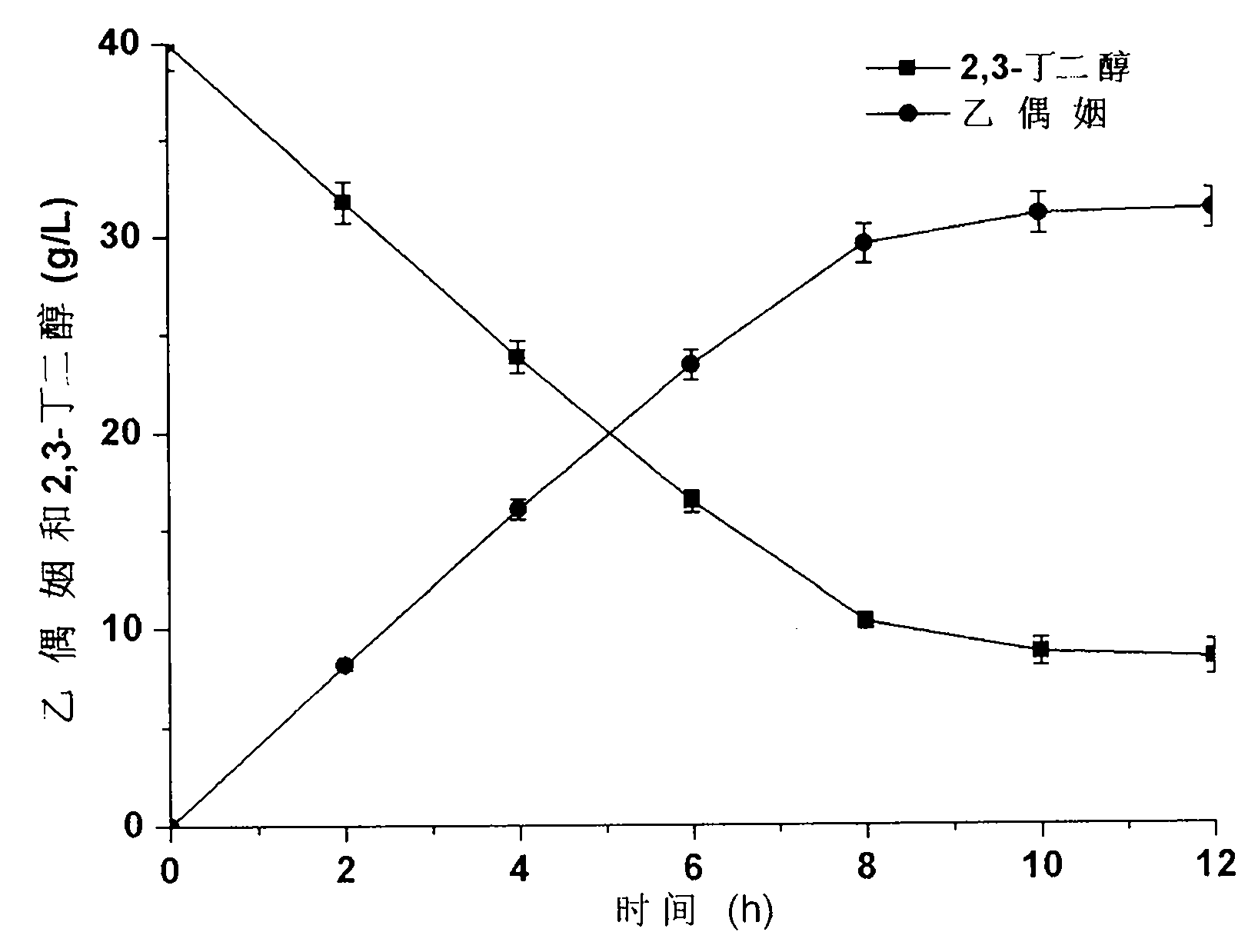
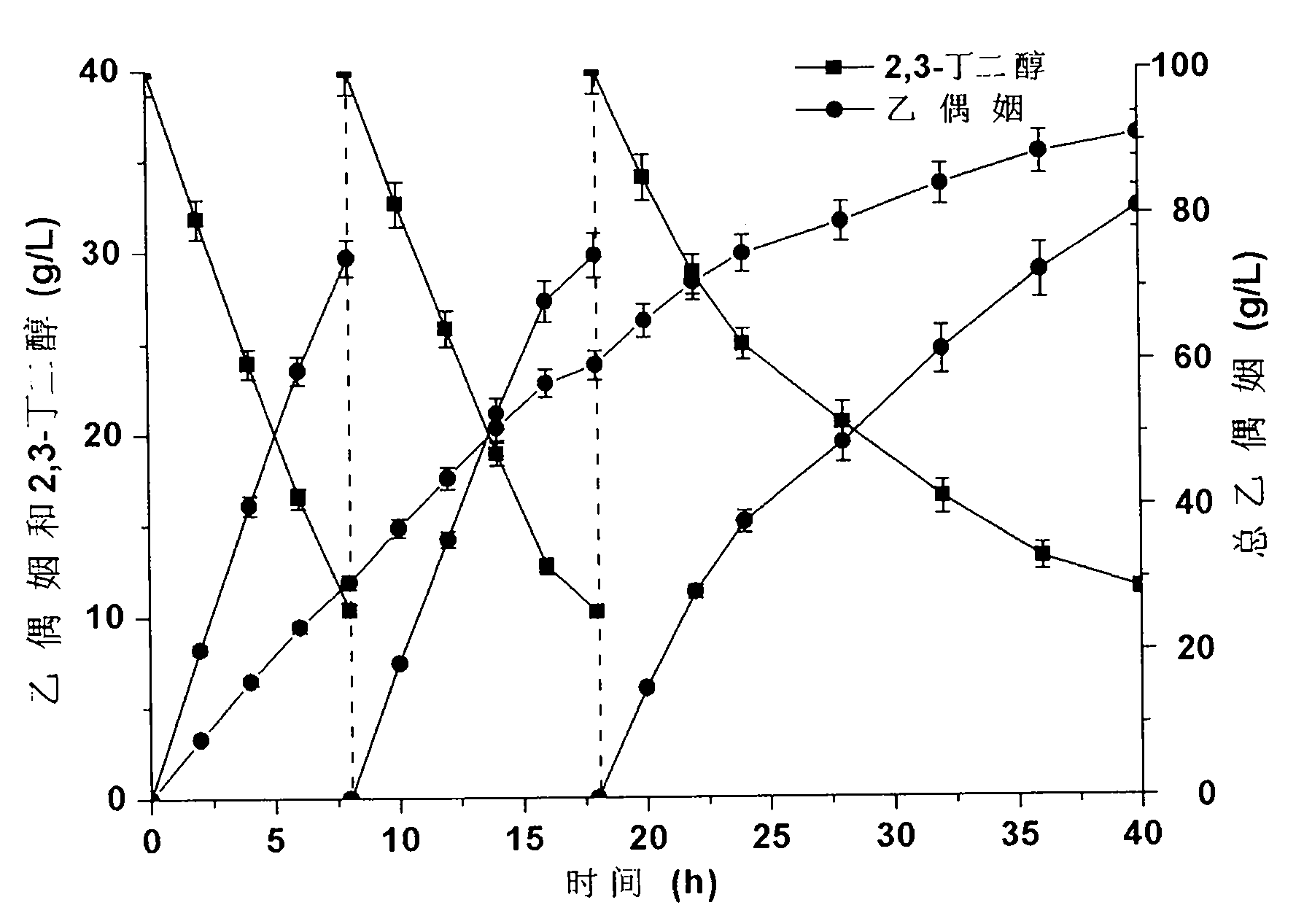
[0034] The acetoin generated by the reaction system was used to detect the transformation activity of the whole cells. The recombinant cells were suspended in 50 mM phosphate buffer (pH8.0) containing 40 g / L 2,3-butanediol, and reacted on a shaking table at 37 ° C for 20 minutes. Reaction solution is centrifuged 1min with the rotating speed of 12000rpm, measures the content of acetoin in the supernatant, and measuring system is as follows: 0.5mL creatine (0.5%w / v), 0.5mLα-naphthol (5%w / v is dissolved in 95% ethanol), 0.5mL potassium hydroxide (10%w / v), 0.01mL centrifuged supernatant and 3.5mL deionized water, put the measurem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com