PAMP37-PR39 fused antibacterial peptide and preparation method thereof
An antibacterial peptide and antibacterial immunity technology, applied in the field of PAMP37-PR39 fusion antibacterial peptide and its preparation, can solve the problems of increasing disease treatment difficulty, high cytotoxicity of eukaryotic cells, low activity of antibacterial peptide, etc. Efficiently obtain and improve the effect of safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Structural Design and Prediction of Antimicrobial Peptides
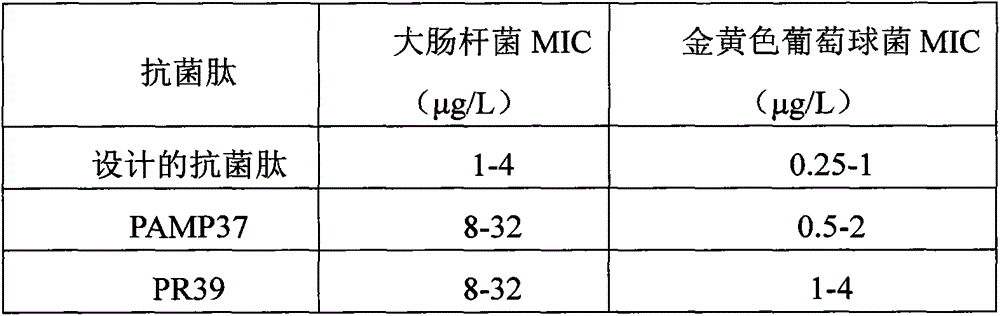
[0033] In order to enhance the bactericidal activity of the antimicrobial peptide, the porcine antimicrobial peptide (PAMP37 and PR39) were constructed in series, and the amino acid sequence of the fusion polypeptide obtained by the construction was SEQ ID1:
[0034] GLLSRLRDFLSDRGRRLGEKIERIGQKIKDLSEFFQSRRRRPPYLPRPRPPPFFPPRLPPRIPPPGFPPRFPPRFP.
[0035] Using the codon preference of Escherichia coli, the recombinant DNA sequence (SEQ ID2) of the sequence was designed, and the N-terminus of the polypeptide was predicted to be an α-helix, and the C-terminus was a linear structure.
Embodiment 2
[0036] Example 2 Construction of Antimicrobial Peptide Expression Vector
[0037] 1) Optimize the DNA sequence to adapt to the codon preference of Escherichia coli, increase the restriction site used for vector construction, and design the fusion polypeptide DNA sequence (SEQ ID3) as follows:
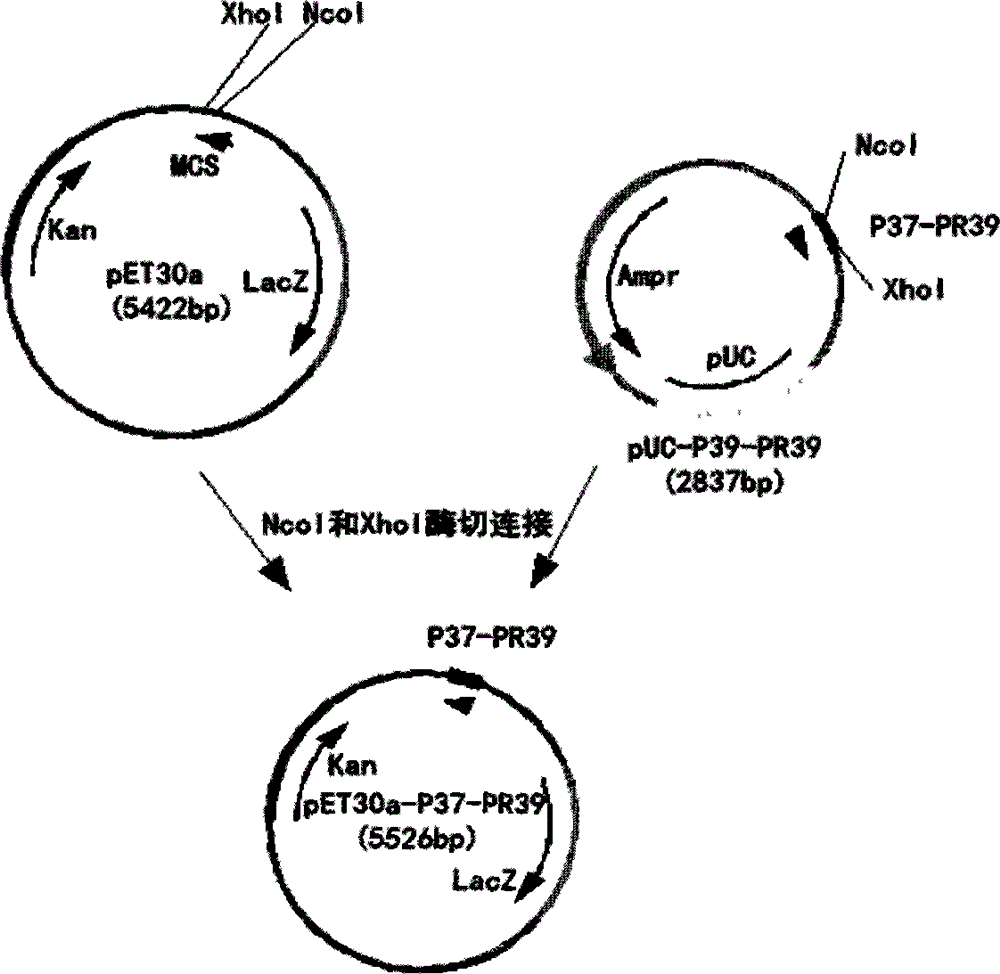
[0038] GGTACC GGTCTGCTGTCTCGTCTGCGTGACTTCCTGTCTGACCGTGGTCGTCGTCTGGGTGAAAAAATCGAACGTATCGGTCAGAAAATCAAAAGACCTGTCTGAATTCTTCCAGTCTCGTCGTCGTCCGCGTCCGCCGTACCTGCCGCGTCCGCGTCCGCCGCCGTTCTTCCCGCCGCGTCTGCCGCCGCGTATACCACCAAGGCTTTCCACCAAGGTTCCCACCACGATTCCCA TAACTCGAG
[0039] The sequences underlined in black are restriction sites (KpnI and XhoI). Sequences are sent to biological companies for synthesis. 3) Construction of antimicrobial peptide expression vector
[0040] The pET30a vector and the synthesized sequence were respectively digested with KpnI and XhoI, and after recovering the digested products, they were ligated to construct the pET30a-P37-PR39 expression vector. The process was as...
Embodiment 3
[0041] Example 3 Induced expression of antimicrobial peptides
[0042] 1) Transform the above expression vector into Escherichia coli BL21 according to conventional methods, spread the transformed bacteria on LB plates, and culture them overnight in an incubator at 37°C. Clearly positive clones were observed growing on the plate.
[0043] 2) Pick a positive single colony and culture it in LB liquid until the OD600 is 0.4-0.6, then add 0.5mM IPTG, induce the expression of the target protein at 250rpm overnight at 37°C, and make a control without adding IPTG.
[0044]3) Collect the bacteria, centrifuge at 10000 g for 15 minutes and discard the supernatant. Sonicate the bacteria in 1M Tris-HCl solution, centrifuge at 20,000g for 30 minutes, analyze the supernatant by SDS-PAGE, and stain the polyacrylamide gel with Coomassie Brilliant Blue to see obvious soluble expression bands of the target protein.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com