Aniline oligomer derivative with electrochromic property and preparation method thereof
An electrochromic and oligomer technology, which is applied in the preparation of organic compounds, carboxylic acid amide preparation, color-changing fluorescent materials, etc., can solve the problems of difficult processing of polyaniline materials, and achieve easy control, simple operation steps, and synthetic routes simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] (1) Preparation of Class A linear aniline oligomer derivatives
[0061] In the present invention, a kind of preparation method of class A linear aniline oligomer derivatives with electrochromic properties is provided, comprising the following steps:
[0062] Step 101: reacting N-phenyl-1,4-p-phenylenediamine and dianhydride of structural formula A-1 in dichloromethane solvent to obtain a compound of structural formula A-2;
[0063] Structural formula used is that the general structural formula of the dianhydride of A-1 is:
[0064]
[0065] Dosage: The molar ratio of N-phenyl-1,4-p-phenylenediamine to dianhydride with structural formula A-1 is 1:1~1:2; the amount of dichloromethane is based on N-phenyl-1, The molar concentration of 4-p-phenylenediamine is determined, and the molar concentration is 0.1-0.5mol / L;
[0066] Reaction conditions: under the conditions of mechanical stirring and nitrogen protection, N-phenyl-1,4-p-phenylenediamine and dianhydride with stru...
Embodiment 1
[0100] In the present invention, a kind of preparation method of class A linear aniline oligomer derivatives with electrochromic properties is provided, comprising the following steps:
[0101] Step 101: Reacting N-phenyl-1,4-p-phenylenediamine and the dianhydride of the structural formula A-1 in dichloromethane solvent to obtain the compound of the structural formula A-2.
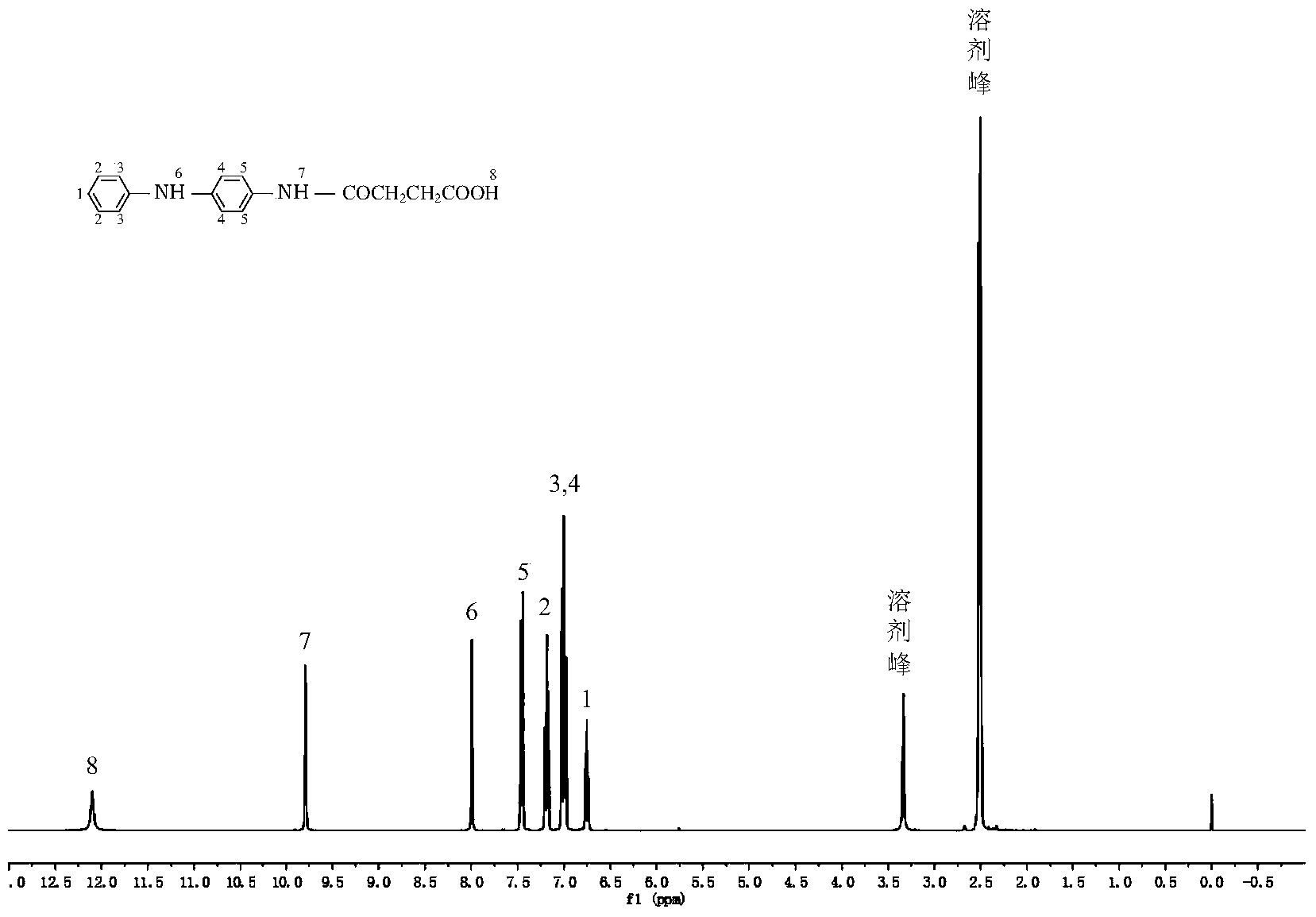
[0102] Take a 250mL three-neck flask and install mechanical stirring, nitrogen protection, add 4.61g (0.025mol) of N-phenyl-1,4-p-phenylenediamine, 2.5g (0.025mol) of succinic anhydride and 150mL of dichloromethane, At a stirring speed of 120 rev / min, as the reaction progressed, a gray precipitate was produced, stirred at room temperature for 240 minutes, and after the reaction was completed, filtered to obtain a filter cake; the filter cake was washed with ether until the filtrate was colorless; finally, the washed The filter cake was treated at a vacuum drying temperature of 40°C for 24 hours to obtain a...
Embodiment 2
[0122] In the present invention, a kind of preparation method of class A linear aniline oligomer derivatives with electrochromic properties is provided, comprising the following steps:
[0123] Step 101: Reacting N-phenyl-1,4-p-phenylenediamine and the dianhydride of the structural formula A-1 in dichloromethane solvent to obtain the compound of the structural formula A-2.
[0124] Take a 500mL three-neck flask, install mechanical stirring, nitrogen protection, add 9.21g (0.050mol) of N-phenyl-1,4-p-phenylenediamine, 8.15g (0.055mol) of phthalic anhydride and 350mL of dichloro Methane, at a stirring speed of 75 rpm, stirred at room temperature for 300 minutes, after the reaction was completed, filtered to obtain a filter cake; then the filter cake was washed with ether until the filtrate was colorless; then the washed filter cake was dried at a vacuum temperature It was treated at 50°C for 48 hours to obtain a compound with the general structural formula A-1, denoted as A03. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Coloring time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com