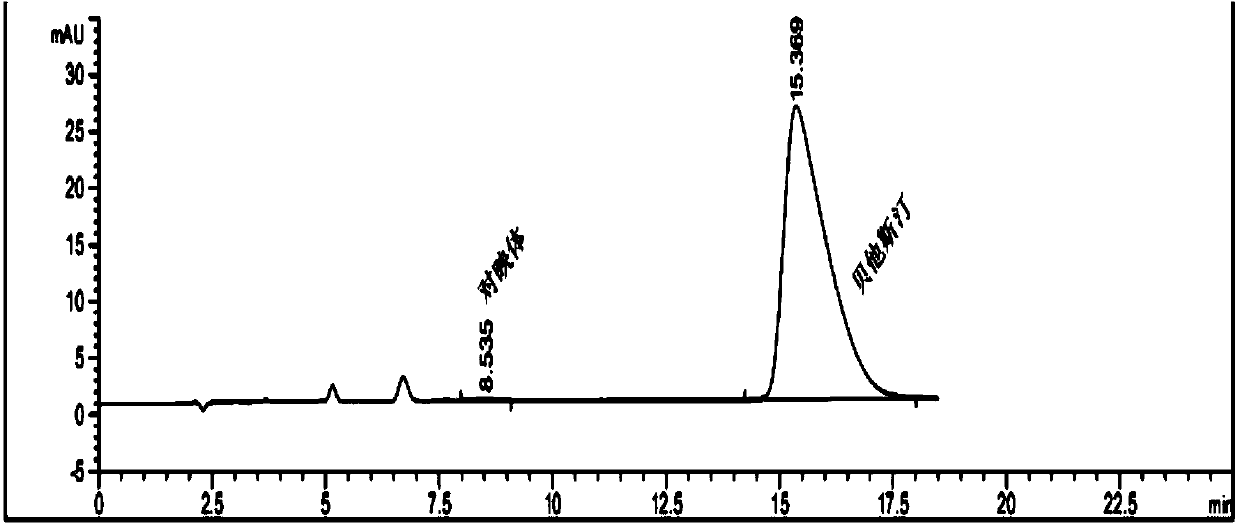
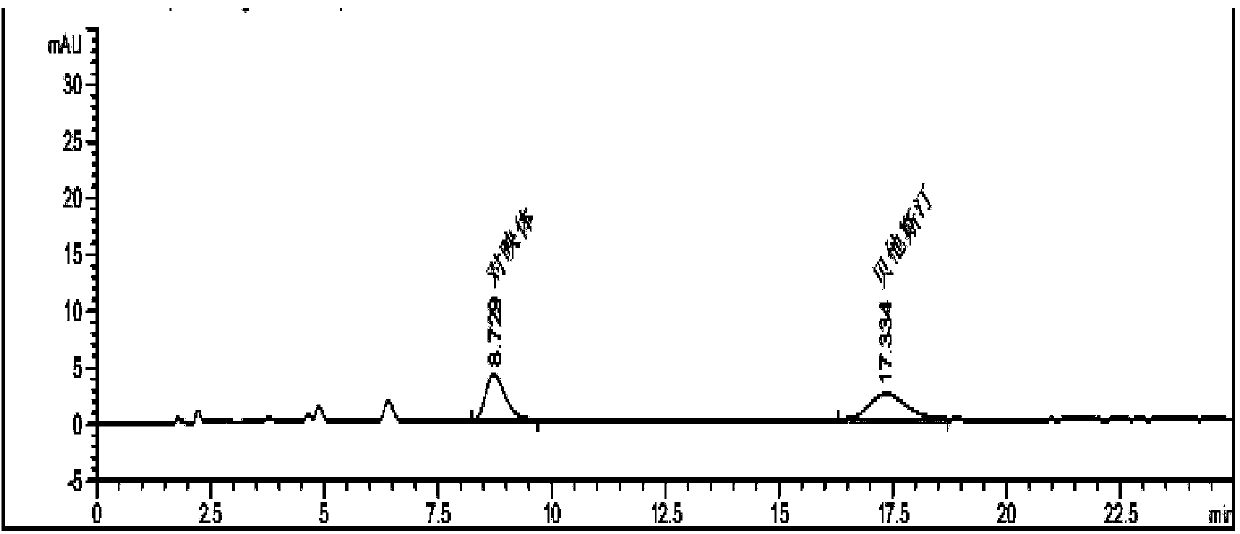
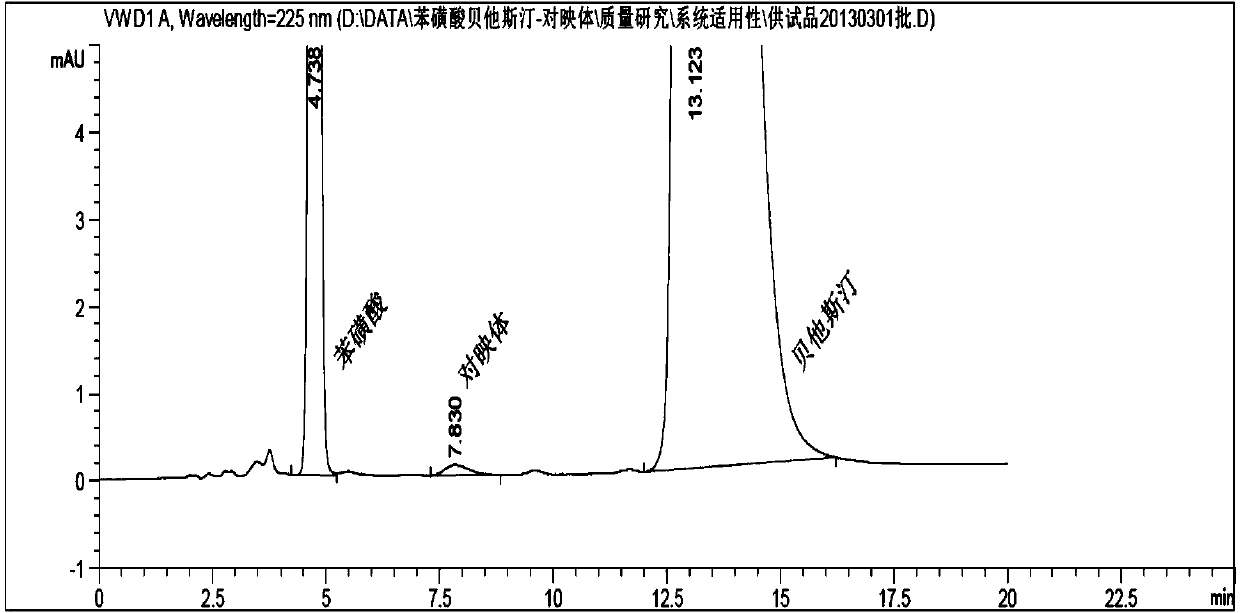
Industrial preparation method for bepotastine besilate or racemoid of bepotastine besilate
A compound and racemization technology, applied in the chemical field, can solve the problems of long reflux time, low condensation reaction yield, high cost, etc., and achieve the effect of simple operation, avoiding heterogeneous reaction, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The application provides an industrial preparation method of bepotastine or a racemic compound thereof, the method comprising the following steps:
[0032] a. Condensation reaction: Dissolve 2-[4-[(S)-(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine (formula (II)) or its racemate in organic In the solvent; add alkaline acid-binding agent, ethyl 4-bromobutyrate (formula (Ⅲ)) to carry out condensation reaction;
[0033] b. Post-treatment: After the reaction is completed, it is extracted with ethyl acetate, washed with lye, and processed to obtain the intermediate (4-[4-[(S)-(4-chlorophenyl)pyridin-2-ylmethoxy]piperidine -1-yl]butanoic acid ethyl ester or its racemate 4-[4-[(4-chlorophenyl)pyridin-2-ylmethoxy]piperidin-1-yl]butanoic acid ethyl ester);
[0034] c. Ester hydrolysis: Add lye to the intermediate for hydrolysis, adjust the solution to acidity, then add dichloromethane, and process to obtain bepotastine (formula (I)) or its racemic compound
[0035] Prefera...
Embodiment 1
[0047] Embodiment 1: Preparation of ethyl 4-[4-[(S)-(4-chlorophenyl)pyridin-2-ylmethoxy]piperidin-1-yl]butanoate:
[0048] Dissolve 2kg of 2-[4-[(S)-(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine in 20L of acetone, add 1.6kg of ethyl 4-bromobutyrate, grind Potassium carbonate 1.2kg, heated to reflux under stirring, reacted for 12 hours, followed by TLC detection, until the raw material point disappeared, the reaction solution was cooled to room temperature, and filtered to remove insoluble matter. The organic layer was concentrated under reduced pressure at 40°C to obtain an oil. Add 20L of purified water to the oil, stir at room temperature for 0.5h, extract with 30L*3 ethyl acetate, separate the layers, wash the organic layer with 11L of 5% sodium hydroxide solution, and dry with 5kg of anhydrous sodium sulfate for 2h. The desiccant was filtered off, concentrated under reduced pressure to obtain 2.0 kg of oily ethyl 4-[4-[(S)-(4-chlorophenyl)pyridin-2-ylmethoxy]piperidin...
Embodiment 2
[0049] Embodiment 2: Preparation of 4-[4-[(4-chlorophenyl)pyridin-2-ylmethoxy]piperidin-1-yl]butanoic acid ethyl ester:
[0050] Dissolve 2kg of 2-[4-[(4-chlorophenyl)(4-piperidinyloxy)methyl]pyridine in 20L of ethanol, add 1.6kg of ethyl 4-bromobutyrate, grind 1.0kg of sodium carbonate , heated to reflux under stirring, reacted for 37 hours, followed by TLC detection, until the raw material point disappeared, the reaction solution was cooled to room temperature, and the insoluble matter was removed by filtration. The organic layer was concentrated under reduced pressure at 40°C to obtain an oil. Add 20L of purified water to the oil, stir at room temperature for 0.5h, extract with 30L*3 ethyl acetate, separate the layers, wash the organic layer with 11L of 5% sodium hydroxide solution, and dry with 5kg of anhydrous sodium sulfate for 2h. The desiccant was filtered off and concentrated under reduced pressure to obtain 2.1 kg of oily ethyl 4-[4-[(4-chlorophenyl)pyridin-2-ylmeth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com