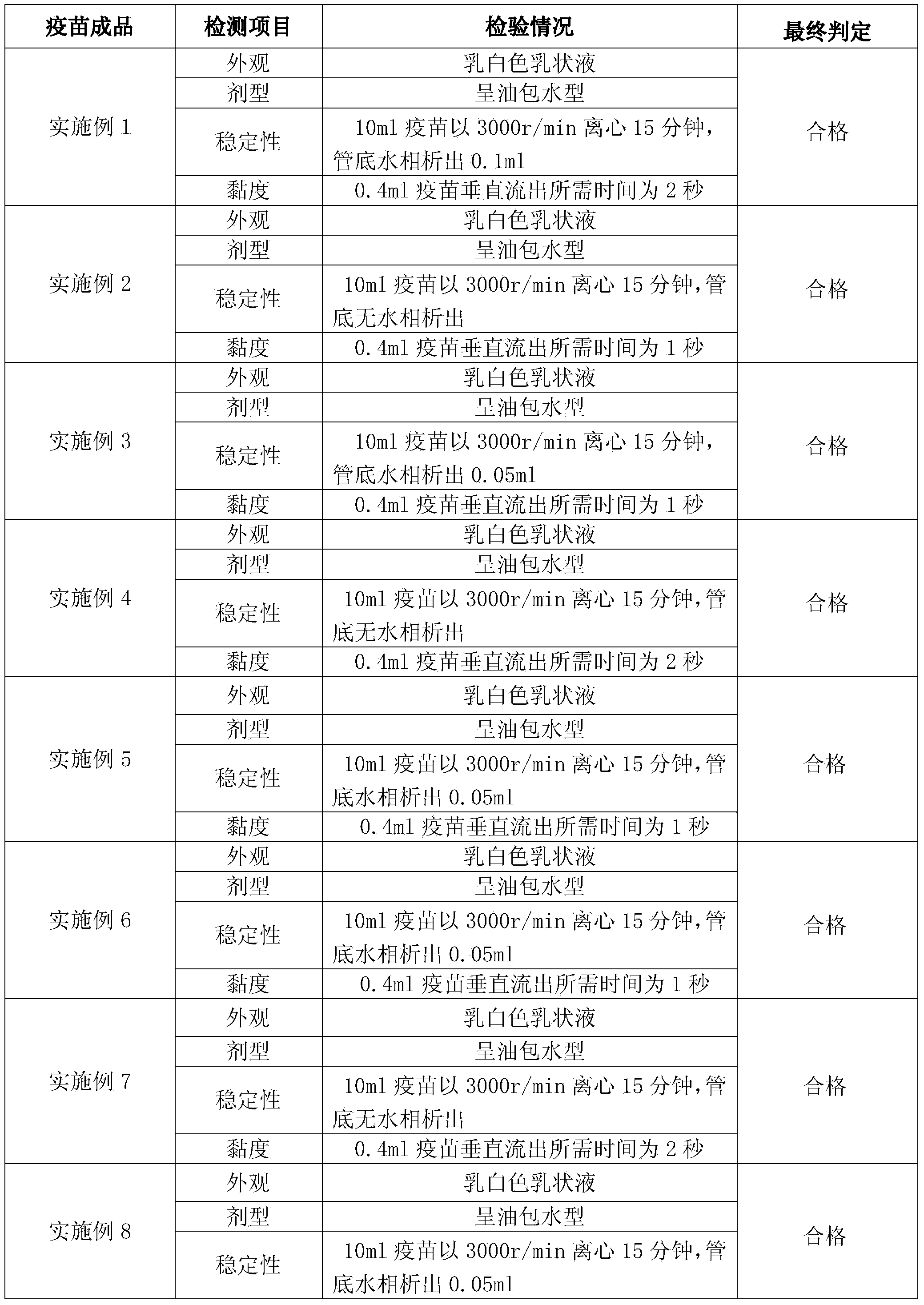Process for preparing avian influenza inactivated vaccine by full suspended culture cells and product
A technology for culturing cells and inactivating vaccines, applied in the field of vaccine preparation, can solve problems such as inability to meet, and achieve the effects of low cost, high pollution rate, and increased culture density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The preparation process of the present invention will be described in detail below.
[0046] (1) Full suspension culture of MDCK cells
[0047] (1.1) Shake flask suspension culture of MDCK cells
[0048] Take out two suspensions of MDCK cells frozen in liquid nitrogen, and after thawing quickly, directly add the suspended nutrient solution to 200ml in a 500ml shaker flask, and place them in suspension culture at 36-37°C. After the cells grow for 60-72 hours, the ratio of 1:3 Passage to other shake flasks at a ratio of ~1:5, and amplify to the required volume of the reactor;
[0049] (1.2) Reactor suspension culture of MDCK cells
[0050] (1.2.1) MDCK cell suspension culture in reactors above 20L
[0051] Dilute the MDCK cells cultured in shake flask suspension in step (1) to 0.5×10 6 After each / ml, inoculate into a bioreactor of more than 20L (20L refers to the working volume, all reactor volumes mentioned below are working volumes, and the working volume of the rea...
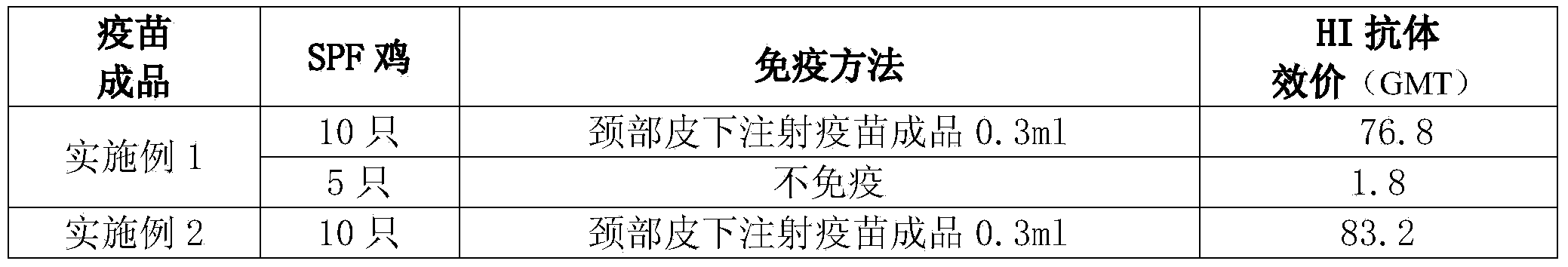
Embodiment 1
[0066] Full suspension culture cell manufactures avian influenza inactivated vaccine, comprises the following steps:
[0067] (1) Full suspension culture of MDCK cells
[0068] (1.1) Shake flask suspension culture of MDCK cells
[0069] Take out two suspended MDCK cells cryopreserved in liquid nitrogen, and after rapid thawing, directly add the suspension nutrient solution to 200ml in a 500ml shake flask, place it in a suspension culture at 37°C, and after the cells grow for 60 hours, pass to the In other shake flasks, amplify to the required volume of the reactor;
[0070] (1.2) Reactor suspension culture of MDCK cells
[0071] (1.2.1) MDCK cell suspension culture in 20L reactor
[0072] Dilute the MDCK cells cultured in shake flask suspension in step (1.1) to 0.5×10 6 cells / ml and inoculated into a 20L bioreactor, supplemented with suspension nutrient solution, the cell culture temperature was set to 36°C, the pH value was 7.2, and the dissolved oxygen was 40%; the cells...
Embodiment 2
[0086] Full suspension culture cell manufactures avian influenza inactivated vaccine, comprises the following steps:
[0087] (1) Full suspension culture of MDCK cells
[0088] (1.1) Shake flask suspension culture of MDCK cells
[0089] Take out two suspended MDCK cells cryopreserved in liquid nitrogen, and after rapid thawing, directly add the suspension nutrient solution to 200ml in a 500ml shake flask, place it in a suspension culture at 37°C, and after the cells grow for 72 hours, pass passage to In other shake flasks, amplify to the required volume of the reactor;
[0090] (1.2) Reactor suspension culture of MDCK cells
[0091] (1.2.1) MDCK cell suspension culture in 20L reactor
[0092] Dilute the MDCK cells cultured in shake flask suspension in step (1.1) to 0.5×10 6 cells / ml and inoculated into a 20L bioreactor, supplemented with suspension nutrient solution, the cell culture temperature was set at 36°C, the pH value was 7.2, and the dissolved oxygen was 60%; the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com