Method for detecting classical swine fever virus specific antibody in pig saliva
A swine fever virus and specific technology, applied in the biological field, can solve the problems of small sample size, low proportion of pig herds, and high animal stress, and achieve convenient transportation and storage, save sampling time, and strong resistance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
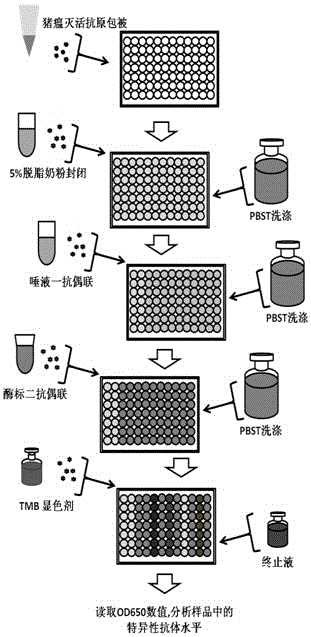
[0022] 1) Preparation, inactivation and antigen-coating of 96-well polystyrene reaction plate for whole swine fever virus antigen;
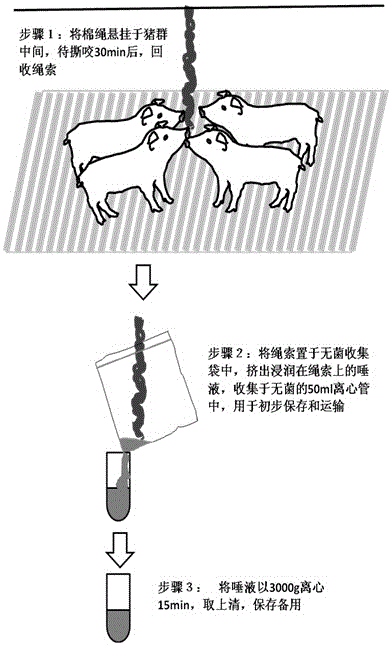
[0023] 2) Use the hanging cotton rope method to induce animals to chew, collect saliva, obtain the primary antibody of saliva after processing, and save it for later use;
[0024] 3) After diluting the saliva primary antibody 1:1 with PBST solution, add it to the reaction wells of the antigen-coated 96-well polystyrene reaction plate according to the number, add 100ul to each reaction well, and react at 22-28°C with saturated humidity for 1 hours, wash and set aside;
[0025] 4) Dilute horseradish peroxidase-labeled goat anti-pig IgG-FC antibody at 1:2000 with PBST solution, and add it to the reaction wells of a 96-well polystyrene reaction plate in sequence, adding 100ul to each reaction well , 22-28 ℃ saturated humidity and dark reaction for 30 minutes, washed with PBST for later use;
[0026] 5) After mixing TMB Chromogenic Agent A and B at ...
Embodiment
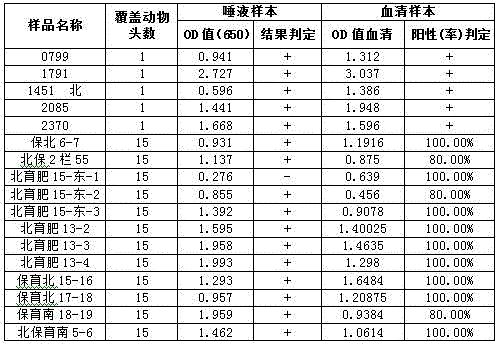
[0032] 1) Infect ST cells with the commercialized CSFV vaccine C strain at a concentration of 1000 TCID50 / ml (half cell infection dose / ml). After culturing for 48 hours, collect the virus liquid, centrifuge at 4000g for 10min, and take the supernatant; centrifuge at 35000g After 1 h, the purified virus was obtained and the TCID50 was titrated. Put the virus liquid in a 65°C water bath and inactivate it for 30 minutes to obtain the purified inactivated antigen of the whole swine fever virus. Dilute the inactivated antigen to 10000 TCID50 / ml with pH 9.6 carbonate buffer, coat 96-well polystyrene ELISA reaction plate at a concentration of 100ul / well, and incubate at 22-28°C for 1h under saturated humidity, then Turn to 4°C and incubate for 16h. Then discard the coating solution, pat dry the ELISA plate, add 5% skimmed milk powder diluted with PBST (pH7.3, 0.05% tween-20) 100ul / well, and block at 22-28°C for 2h. Discard the blocking solution, pat dry the ELISA plate, add 150 ul ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com