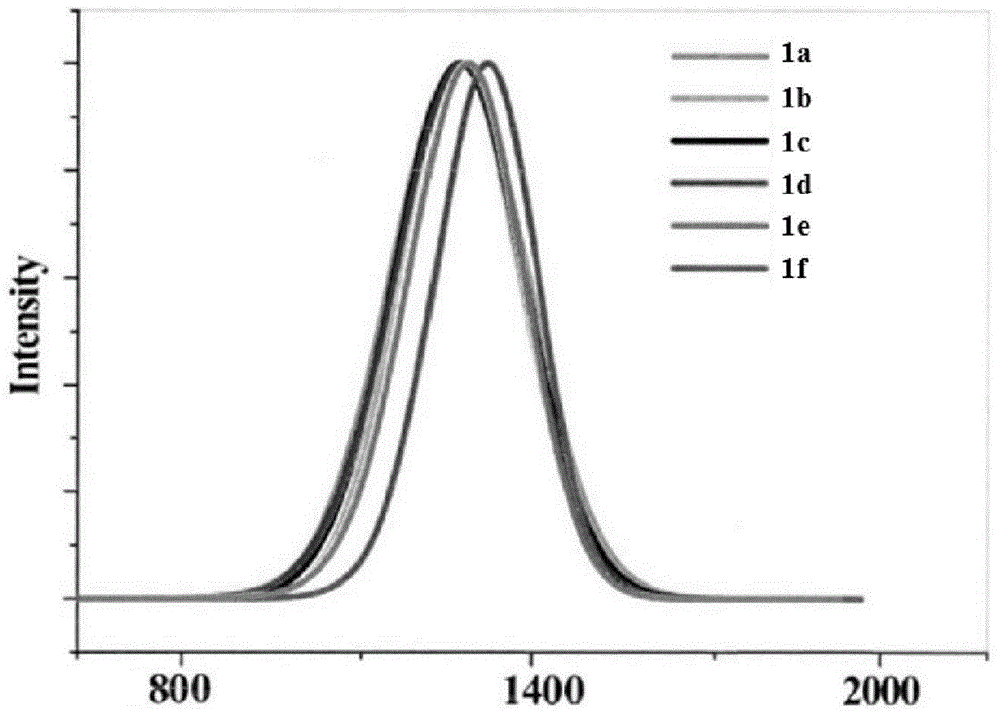
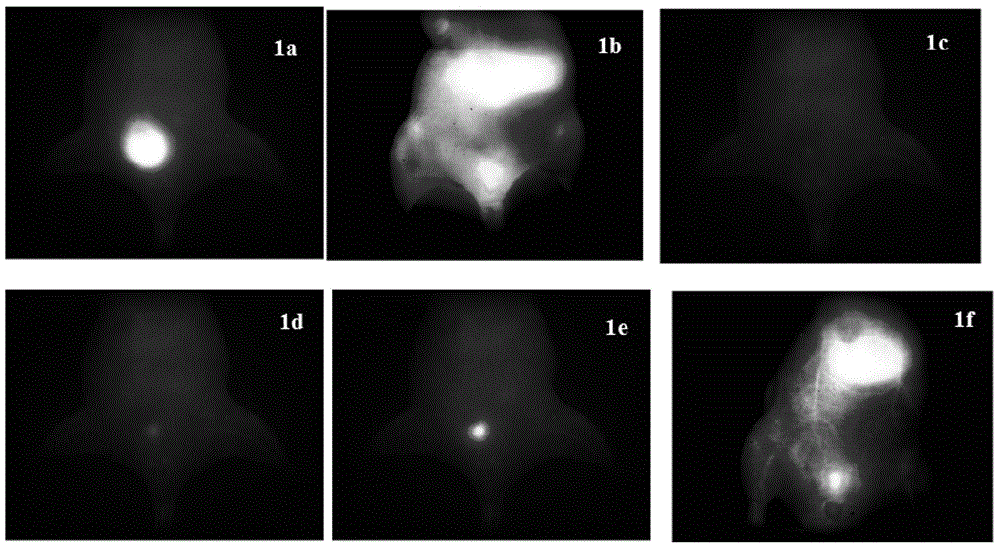
Modifiable fluorescent compound, synthesis method thereof and application of modifiable fluorescent compound as near-infrared II-region reporter molecule
A compound and near-infrared technology, applied in the field of fluorescent compounds, can solve problems such as biocompatibility, poor druggability, rare research reports, and no post-modification, etc., to improve water solubility and biocompatibility, good imaging effect, and broad field The effect of applying the foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: Synthesis (1a)
[0048]
[0049] Take 6.7g (13mmol) of compound (2a), 3.84g (10mmol) of compound (3), 0.5246g (2mmol) of triphenylphosphine, and 2.12g (20mmol) of sodium carbonate into a 50mL round bottom flask. Under nitrogen protection, 30 mL of toluene / water mixture (v / v, 8:2) was added. Nitrogen gas was passed into the reaction liquid, oxygen in the reaction liquid was excluded for 20 min, and tetrakis(triphenylphosphine) palladium 1.1556 g (1 mmol) was added, and nitrogen gas was continuously passed into the reaction liquid for 10 min. Under the protection of nitrogen, the reaction was heated to reflux for 24h. After the reaction, ethyl acetate (EA) (15 mL×3) was added for extraction three times, the organic phases were combined and washed twice with water (10 mL×2). The organic phase was dried with anhydrous magnesium sulfate for 1 h, filtered, and the filtrate was spin-dried and passed through a silica gel column to obtain 5.895 g of the reacti...
Embodiment 2
[0052] Embodiment 2: synthesis (1b)
[0053]
[0054] Take compound (2b) 7.53g (13mmol), compound (3) 3.84g (10mmol), triphenylphosphine 0.5246g (2mmol), sodium carbonate 2.12g, 20mmol into a 50mL round bottom flask. Under nitrogen protection, 30 mL of toluene / water mixture (v / v, 8:2) was added. Nitrogen gas was passed into the reaction solution, and the oxygen in the reaction solution was excluded for 20 minutes, then 1.1556 g (1 mmol) of tetrakis(triphenylphosphine) palladium was added, and nitrogen gas was continuously passed into the reaction solution for 10 minutes. Under the protection of nitrogen, the reaction was heated to reflux for 48h. After the reaction was completed, EA (15 mL×3) was added for extraction three times, the organic phases were combined and washed twice with water (10 mL×2). The organic phase was dried with anhydrous magnesium sulfate for 1 h, filtered, and the filtrate was spin-dried and passed through a silica gel column to obtain 6.724 g of a ...
Embodiment 3
[0057] Embodiment 3: synthesis (1c)
[0058]
[0059] Take 9.12g (13mmol) of compound (2c), 3.84g (10mmol) of compound (3), 0.5246g (2mmol) of triphenylphosphine, and 2.12g (20mmol) of sodium carbonate into a 50mL round bottom flask. Under nitrogen protection, 30 mL of toluene / water mixture (v / v, 8:2) was added. Nitrogen gas was passed into the reaction liquid, oxygen in the reaction liquid was excluded for 20 min, and tetrakis(triphenylphosphine) palladium 1.1556 g (1 mmol) was added, and nitrogen gas was continuously passed into the reaction liquid for 10 min. Under the protection of nitrogen, the reaction was heated to reflux for 6h. After the reaction was completed, EA (15 mL×3) was added for extraction three times, the organic phases were combined and washed twice with water (10 mL×2). The organic phase was dried with anhydrous magnesium sulfate for 1 h, filtered, and the filtrate was spin-dried and passed through a silica gel column to obtain 6.94 g of a reaction in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com