Nesting method for pregabalin intermediate mother liquor
A technology of intermediates and mother liquors, applied in the field of application of pregabalin intermediate mother liquors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
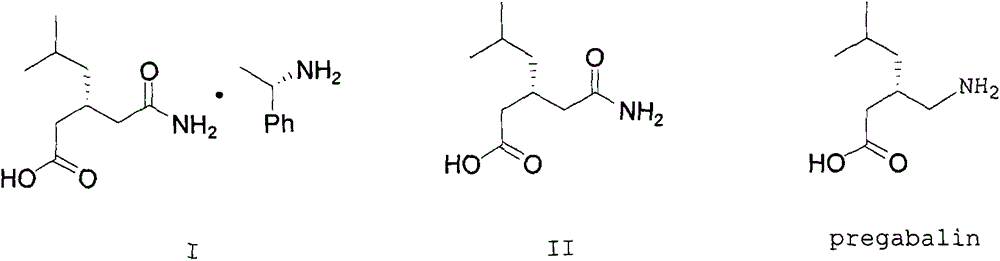
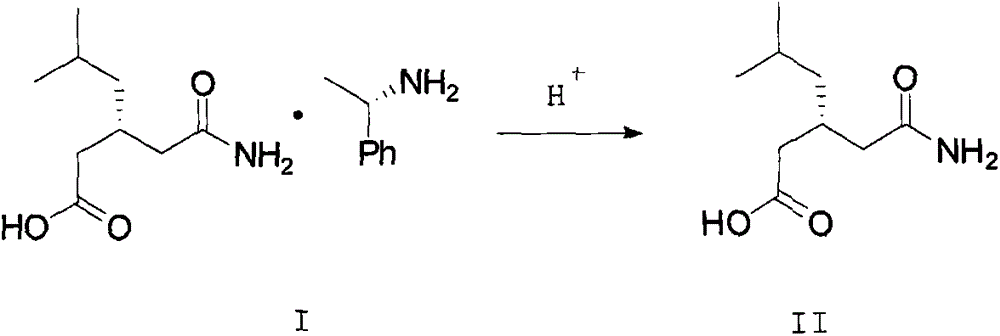
[0029] Add (R)-(-)-3-(carbamoylmethyl)-5-methylhexanoic acid-(R)-(+)-α-phenethylamine salt 200g into a 1000ml four-neck flask, free mother liquor 600g and 40g water, heat up to 40°C and stir mechanically. When the internal temperature of the system reaches 40°C, keep stirring for 10 minutes, cool down to 25°C, start to slowly add 77g of concentrated hydrochloric acid, adjust the pH to 1.5, start to cool down to 5°C, and keep warm Stir for 3 hours, filter with suction and dry to obtain 132.6 g of (R)-(-)-3-(carbamoylmethyl)-5-methylhexanoic acid with a yield of 109.2% and a purity of 99.6%. 0.11%.
example 2
[0031] Add (R)-(-)-3-(carbamoylmethyl)-5-methylhexanoic acid-(R)-(+)-α-phenethylamine salt 200g into a 1000ml four-neck flask, free mother liquor 640g and 40g methanol, heat up to 35°C and stir mechanically, when the internal temperature of the system reaches 35°C, keep stirring for 10 minutes, start to slowly add 77g concentrated hydrochloric acid dropwise, adjust the pH to 1.0, start to cool down to 8°C, keep stirring for 4 hours, shake Filtration and drying gave 127.5 g of (R)-(-)-3-(carbamoylmethyl)-5-methylhexanoic acid, with a yield of 105.0%, a purity of 99.6%, and an isomer of 0.12%.
example 3
[0033] Add (R)-(-)-3-(carbamoylmethyl)-5-methylhexanoic acid-(R)-(+)-α-phenethylamine salt 200g into a 1000ml four-neck flask, free mother liquor 550g and 40g isopropanol, heat up to 45°C and stir mechanically. When the internal temperature of the system reaches 45°C, keep stirring for 10 minutes, then cool down to 30°C, start to slowly add 77g of concentrated hydrochloric acid dropwise, adjust the pH to 1.0, and start to cool down to 5 ℃, heat preservation and stirring for 3.5 hours, rejection filtration, and drying to obtain (R)-(-)-3-(carbamoylmethyl)-5-methylhexanoic acid 126.4g, yield 104.1%, purity 99.4%, Isomer 0.15%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com