A biphenylurea compound containing an oxime group and its preparation method and application
A compound, the technology of bifenurea, applied in the field of bifenurea compound and its preparation, and anti-tumor compound, can solve the problems that chemical drugs cannot achieve therapeutic effect, hair loss, etc., achieve inhibition of tumor growth and migration, and enhance inhibitory activity , good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
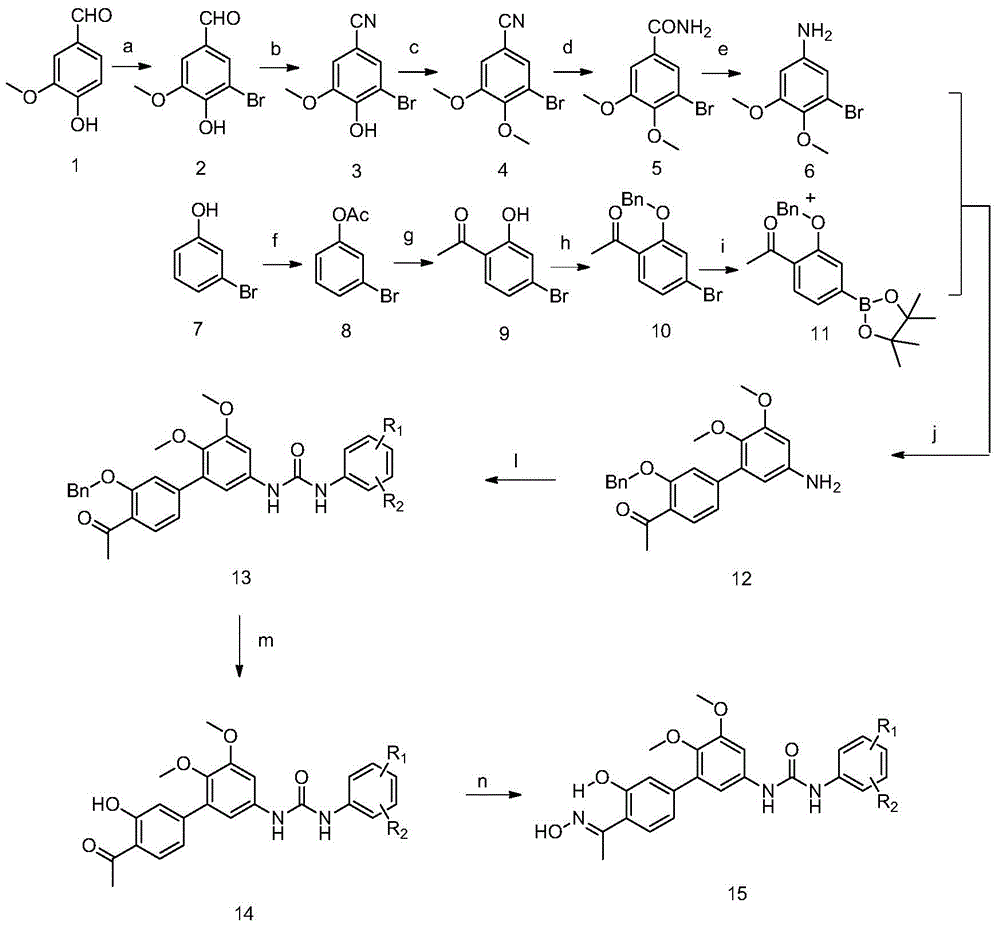
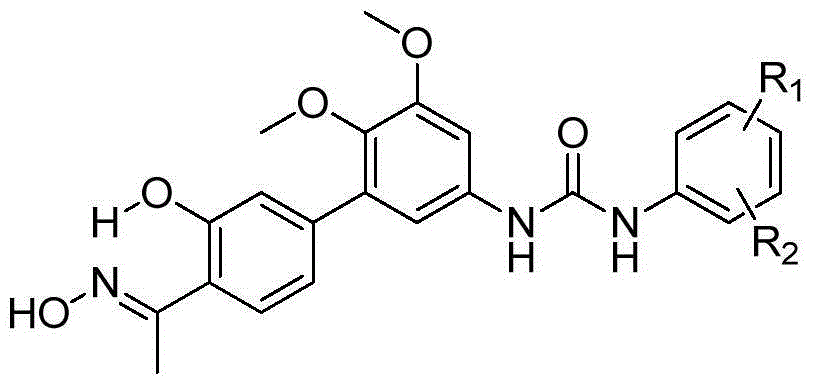
[0052] In the structural formula of this compound, R 1 for hydrogen, R 2 It is an alkoxy group with 2 carbon atoms, and the end is substituted by diethylamino, prepared by the following steps (see figure 1 ):
[0053] 1) 3-methoxyl-4-hydroxybenzaldehyde (1) is prepared by bromination reaction compound 3-methoxyl-4-hydroxyl-5-bromobenzaldehyde (2)
[0054] 20.0g (132mmol) of 3-methoxy-4-hydroxybenzaldehyde (1), 21.59g (263mmol) of sodium acetate and 0.68g (12mmol) of iron powder were placed in a 500mL three-necked flask, and 120mL of glacial acetic acid was added, Stir at room temperature for 30 minutes; after the stirring is completed, control the temperature at 23-25°C, add dropwise a solution prepared by mixing 7.0mL (140mmol) liquid bromine and 30mL glacial acetic acid in advance, and then control the temperature at 23-25°C to continue Stir for 3h;
[0055] Then add 250 mL of ice water, stir for 1 h; filter, dry the solid, and recrystallize from ethanol to obtain 24.70 ...
Embodiment 2
[0102] where R 1 is methyl, R 2 It is an alkoxy group with 3 carbon atoms, the terminal is substituted by dimethylamino, and it is located at the para position of urea.
[0103] Steps 1 to 8 are the same as those in Example 1, that is, compound 1-(4'-acetyl-5,6-dimethoxy- 3'-Hydroxy-[1,1'-biphenyl]-3-yl)-3-(4-(2-(dimethylamino)propyl)phenyl)urea followed by conversion of acetyl group to oxime group, The specific operation steps are:
[0104] 0.51g (1mmol) 1-(4'-acetyl-5,6-dimethoxy-3'-hydroxy-[1,1'-biphenyl]-3-yl)-3 -(4-(2-(Dimethylamino)propyl)phenyl)urea dissolved, heated to 50°C, added 2mL of 0.72g (10mmol) hydroxylamine hydrochloride aqueous solution, reacted for 1h, cooled the reaction solution to room temperature, and Saturated sodium carbonate solution was added to the reaction solution to adjust the reaction solution to pH=8, extracted with chloroform (20mL×3), the organic phases were combined, and dried over anhydrous sodium sulfate to obtain 0.47g of crude produc...
Embodiment 3
[0110] where R 1is methyl, R 2 The compound whose tertiary amino group is morpholino, n=3, is located at the meta-position of urea.
[0111] Steps 1 to 8 are the same as those in Example 1, that is, compound 1-(4'-acetyl-5,6-dimethoxy- 3'-Hydroxy-[1,1'-biphenyl]-3-yl)-3-(2-methyl-5-(2-morpholinopropyl)phenyl)urea followed by conversion of the acetyl group to oxime base, the specific operation steps are:
[0112] 0.56g (1mmol) 1-(4'-acetyl-5,6-dimethoxy-3'-hydroxyl-[1,1'-biphenyl]-3-yl)-3 -(2-Methyl-5-(2-morpholinopropyl)phenyl)urea dissolved, heated to 50°C, added 0.72g (10mmol) of hydroxylamine hydrochloride aqueous solution 2mL, reacted for 1h, and cooled the reaction solution to room temperature , adding saturated sodium carbonate solution to the reaction solution to adjust the reaction solution to pH=8, extracting with chloroform (20mL×3), combining the organic phases and drying with anhydrous sodium sulfate to obtain 0.50g of crude product with a yield of 87.3%. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com