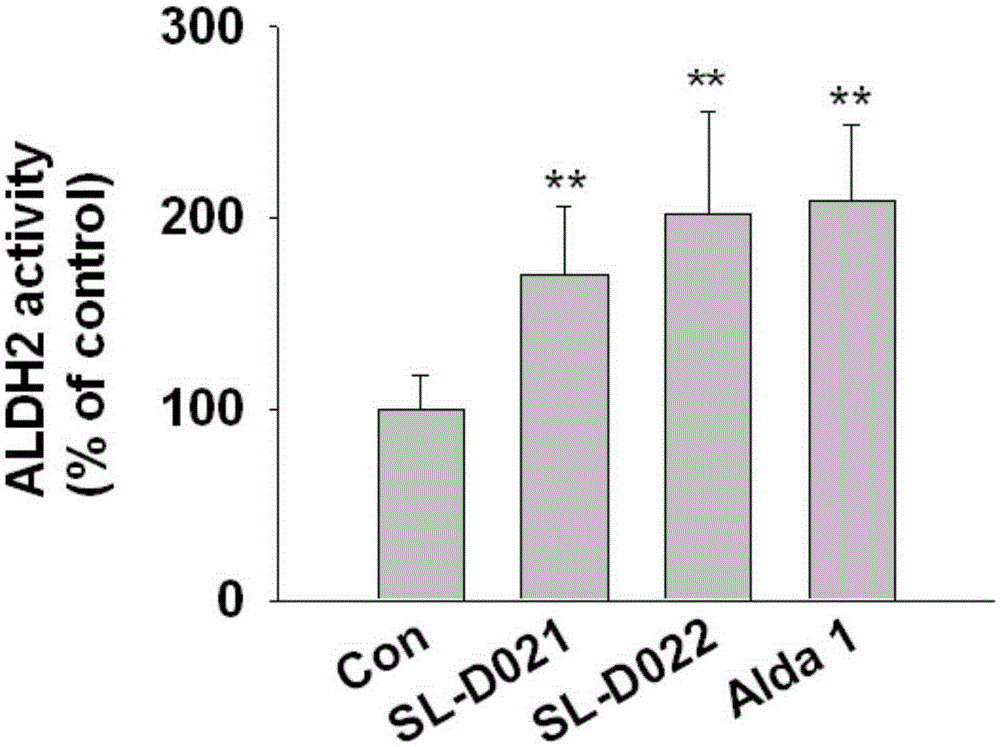
Two kinds of acetaldehyde dehydrogenase agonists, their preparation method and application
A technology for agonists and compounds, applied in the field of medicine, can solve the problems of loss and decrease of ALDH2 enzyme activity, and achieve the effects of simple preparation method, cheap and easy-to-obtain raw materials, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of 3,4-methylenedioxybenzylamine:
[0033] Add 300ml of anhydrous isopropyl ether to a 250ml three-necked bottle, cool to -5°C to 0°C, add 12g (90mmol) of aluminum trichloride and 5.2g (136mmol) of lithium aluminum tetrahydrogen, stir for 5 minutes, and control the temperature Between -5°C and 0°C, a mixed solution of 10 g (68 mmol) of 3,4-(methylenedioxy)benzonitrile and 300 ml of isopropyl ether was added dropwise. The reaction mixture was stirred for 10 hours and quenched with methanol at 0°C. The reaction mixture was basified with 1M aqueous NaOH and extracted with ethyl acetate. The organic layers were combined, washed with water and saturated sodium bicarbonate solution, dried over anhydrous magnesium sulfate, ethyl acetate was evaporated under normal pressure, vacuum pump vacuum distillation, 9.1 g of 10 mmHg fraction at 136-138 °C were collected, yield 88.5%. 1H NMR (300MHz, DMSO) 1.73 (brs, 2H), 3.62 (s, 2H), 5.95 (s, 2H), 6.74-6.81 (m, 2H), 6.91 ...
Embodiment 2
[0035] Preparation of SL-D021:
[0036] Add 20ml of water and 2g (0.0132mol) of 3,4-methylenedioxybenzylamine to a 100ml three-neck flask under stirring, wait until the temperature in the reaction flask drops to 5-10 degrees, stir magnetically for 2 minutes in an ice bath, drop Add 1.93 g (0.0148 mol) of furoyl chloride, and dropwise add acid chloride and sodium hydroxide solution (0.6 g, 0.015 mol of sodium hydroxide plus 2.5 ml of water). After the addition is complete, continue stirring for 30 minutes, and monitor by TLC until the end of the reaction. Stop the reaction, filter, and wash the filter cake successively with 3ml×2 water, 3ml×2 water, 5% HCl 5ml, and 3ml×2 water to obtain a crude product. The crude product was recrystallized from methanol to obtain 2.9 g of pure product. Yield 91%, mp: 114-116°C. 1HNMR (300MHz, CDCl 3 )4.51(d,J=5.7Hz,2H),5.94(s,2H),6.48-6.50(m,1H),6.66(brs,1H),6.75-6.79(m,2H),6.82-6.84(m ,1H), 7.13(d,J=3.6Hz,1H),7.41(d,J=0.9Hz,1H). HRMS:m / z2...
Embodiment 3
[0038] Preparation of SL-D022:
[0039] Add 20ml of water and 2g (0.0132mol) of 3,4-methylenedioxybenzylamine to a 100ml three-neck flask under stirring, wait until the temperature in the reaction flask drops to 5-10 degrees, stir magnetically for 2 minutes in an ice bath, drop Add 2.35g (0.0148mol) of 3-fluorobenzoyl chloride, and dropwise add acid chloride and sodium hydroxide solution (0.6g 0.015mol of sodium hydroxide plus 2.5ml of water). end point of the reaction. Stop the reaction, filter, and wash the filter cake successively with 3ml×2 water, 3ml×2 water, 5% HCl 5ml, 3ml×2 water, and dry the crude product. The crude product was recrystallized from methanol to obtain 2.6 g of pure product. Yield 72%, mp: 124-126°C. 1 HNMR (300MHz, CDCl 3 )4.29(d,J=6.0Hz,2H),5.91(d,J=1.2Hz,2H),6.14(brs,1H),6.64-6.69(m,2H),6.70-6.73(m,2H), 6.92-6.93(m,1H),6.96-6.99(m,1H),7.22-7.27(m,1H).HRMS:m / z274.79(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com