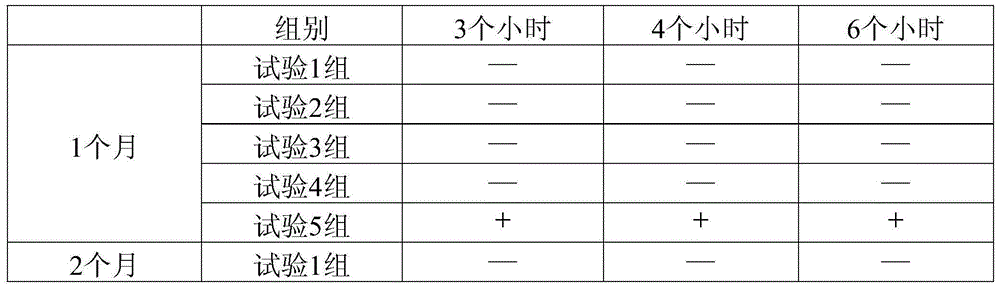
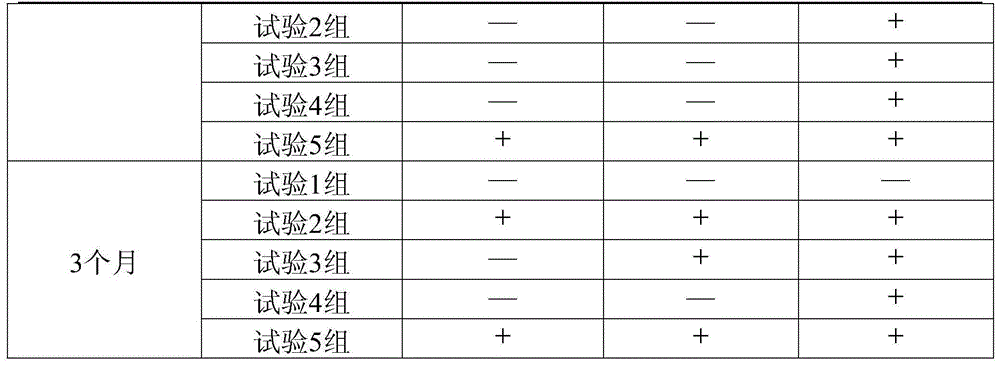
A pharmaceutical composition containing notoginseng and notoginseng saponin r1 and its preparation
A technology of Panax notoginseng saponins and compositions, applied in the field of medicine, can solve problems such as hidden dangers of safety, adverse reactions, and untraceability of doctors and patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of Panax notoginseng:
[0025] (1) Take 50g of Panax notoginseng, dry it at 55°C, and crush it to 120 mesh;
[0026] (2) Add notoginseng powder into 1000 ml of ethanol solution with a concentration of 80% by mass, then add 0.5 g of glycoside hydrolase, stir evenly, and conduct sealed enzymolysis and leaching at 50° C. for 72 hours;
[0027] (3) Filter the reaction solution after enzymolysis, concentrate the filtrate and put it on a 300-mesh polyamide chromatography separation column, first elute with 90% ethanol solution with a mass percentage concentration, then elute with 100% ethanol, and collect 100% ethanol The eluent of ethanol was concentrated to obtain the crude product of notoginseng, and its purity was 60%;
[0028] (4) Dissolve the above-mentioned notoginseng crude product in hot water, put it on a 600-mesh polyamide chromatography column, first elute with 90% ethanol solution with a mass percentage concentration, then elute with 100% ethanol, an...
Embodiment 2
[0030] Preparation of Panax notoginseng:
[0031] (1) Take 50g of Panax notoginseng, dry it at 40°C, and crush it to 100 mesh;
[0032] (2) Add notoginseng powder into 500ml of ethanol solution with a concentration of 40% by mass, and then add 2.5g of glycoside hydrolase, stir evenly, and conduct sealed enzymolysis and leaching at 46°C for 62 hours;
[0033] (3) Filter the reaction solution after enzymolysis, concentrate the filtrate and put it on a 100-mesh polyamide chromatography separation column, first elute with an ethanol solution with a mass percentage concentration of 70%, then elute with 100% ethanol, and collect 100% ethanol The eluent of ethanol is concentrated to obtain the crude product of notoginseng, and its purity is 50%;
[0034] (4) Dissolve the above-mentioned notoginseng crude product in hot water, put it on a 300-mesh polyamide chromatography column, first elute with an ethanol solution with a concentration of 85% by mass percentage, then elute with 100%...
Embodiment 3
[0036] Preparation of Panax notoginseng:
[0037] (1) Take 50g of Panax notoginseng, dry it naturally, and crush it to 90 mesh;
[0038] (2) Add notoginseng powder into 400 ml of ethanol solution with a concentration of 60% by mass, then add 2 g of glycoside hydrolase, stir evenly, and conduct sealed enzymolysis and leaching at 45° C. for 32 hours;
[0039] (3) Filter the reaction solution after enzymolysis, concentrate the filtrate and put it on a 200-mesh polyamide chromatography separation column, first elute with an ethanol solution with a mass percentage concentration of 70%, then elute with 100% ethanol, and collect 100% ethanol The eluent of ethanol was concentrated to obtain the crude product of notoginseng, and its purity was 53.5%;
[0040] (4) Dissolve the above-mentioned notoginseng crude product in hot water, put it on a 400-mesh polyamide chromatography column, first elute with an ethanol solution with a concentration of 87% by mass percentage, then elute with 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com