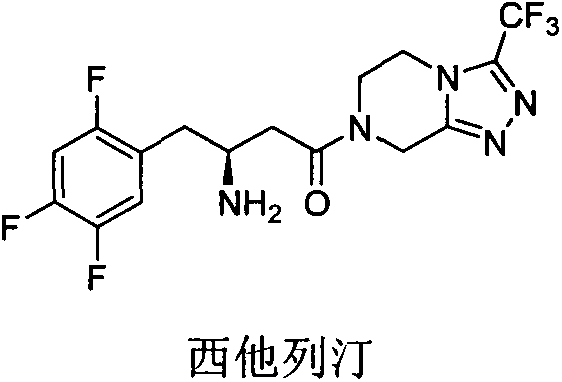
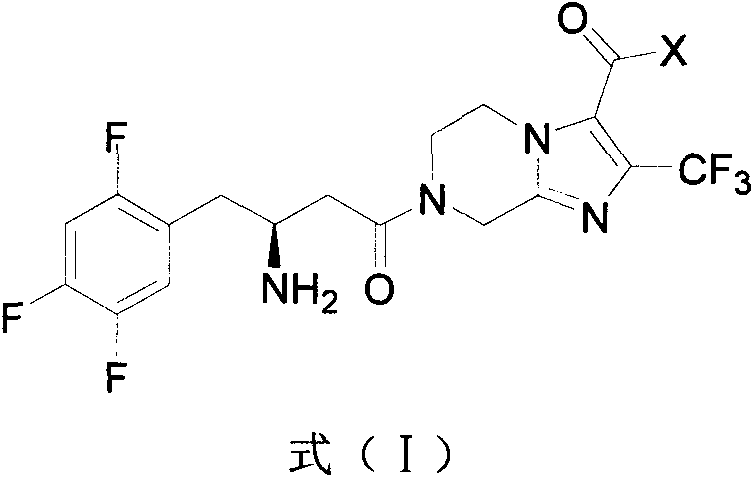
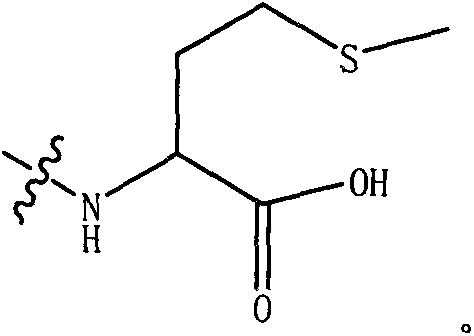
A kind of dpp-4 inhibitor with piperazine structure
A technology of isomers and compounds, applied in the field of organic synthesis, can solve the problems of limited varieties of diabetes and inability to meet clinical needs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Chemical reaction formula:
[0060]
[0061] first step reaction
[0062] Weigh 6.63g of 2-aminopyrazine and 16.78g of ethyl 2-bromotrifluoromethyl acetate and mix them into a 250mL round bottom flask, measure 50mL of ethanol to dissolve and stir evenly, heat and stir at 95°C overnight. TLC detection (EA: MeOH = 20: 1, ammonia water) reaction process, after the raw materials are almost completely reacted, the sample is directly mixed and passed through the column to collect the product point (PE: EA = 20: 1 ~ 1: 1), concentrated to obtain intermediate 1 about 5.5 g, as light yellow solid, the yield is about 30%.
[0063] second step reaction
[0064] Measure about 100mL of MeOH to dissolve 5.5g of intermediate 1, add 1.1g of palladium carbon catalyst, replace with nitrogen three times and then replace with hydrogen three times, heat and stir at 30°C overnight, and seal under normal pressure for hydrogen protection. TLC detection (EA: MeOH = 20: 1, ammonia water) r...
Embodiment 2
[0073] The preparation of embodiment 2 compound 1-2
[0074] Chemical reaction formula:
[0075]
[0076] Dissolve 0.2g of intermediate 4 in 10ml of toluene, then add triphenylphosphine (1.5ep), dropwise add DEAD (1.5ep) under ice-water bath conditions, finally add 2.0ep methanol to the reaction solution, and then Stirring and reacting for 24 hours, TLC detection showed that the reaction of the raw materials was complete, and concentrated under reduced pressure. The obtained product was deprotected by methanol / concentrated hydrochloric acid to obtain about 100 mg of the target product I-2.
[0077] MS: 465[M+H],
[0078] H-NMR: δ7.44-7.35(m, 1H), 7.29-7.26(m, 1H), 5.19-5.06(m, 2H), 4.34-4.30(m, 1H), 4.12-4.02(m, 2H) , 3.94-3.84 (m, 5H), 3.20-2.89 (m, 2H), 2.83-2.81 (m, 2H).
Embodiment 3
[0079] The preparation of embodiment 3 compound I-7
[0080] Chemical reaction formula:
[0081]
[0082] Add 0.50 g of intermediate 4, 10 mL of DMF, 2.0 ep of triethylamine, 1.3 ep of HOBT and 1.5 ep of EDC·HCl into a 100 ml three-necked flask, stir at room temperature for 1 h, add 1.0 ep of methylamine in tetrahydrofuran, and react overnight at 31 ° C. TLC (DCM: MeOH=20:1, 2 drops of ammonia) tracked the completion of the reaction, the reaction solution was poured into 200ml of ice water, a large amount of white solid was precipitated, suction filtered, the filter cake was washed with water, and dried in vacuo at 45°C to obtain 0.55g of a gray solid. The obtained product was de-boc-protected to obtain compound I-7.
[0083] MS: 464[M+H],
[0084] H-NMR: δ7.43-7.35(m, 1H), 7.26-7.24(m, 1H), 5.18-5.06(m, 2H), 4.36-4.31(m, 1H), 4.16-4.02(m, 2H) , 3.97-3.92 (m, 2H), 3.24-2.87 (m, 2H), 2.87-2.82 (m, 2H), 2.75 (s, 3H).
[0085] Compound I-3, Compound I-4, and Compound I-5 wer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com