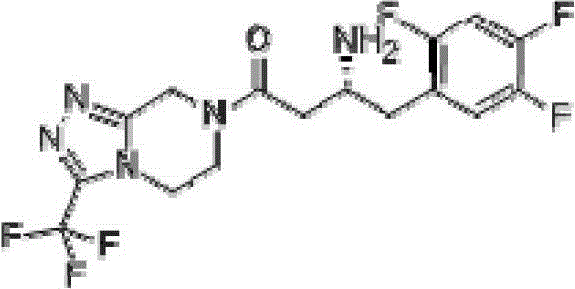
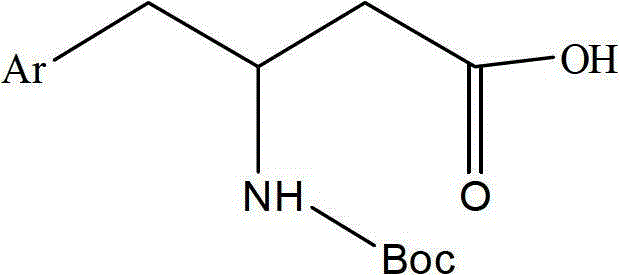
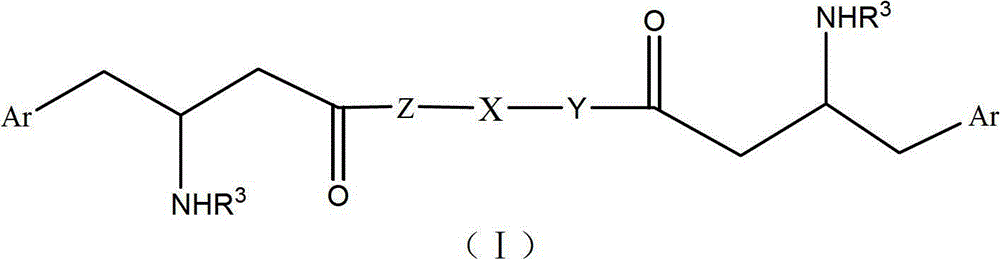dpp-4 inhibitor compound
A compound, n-b- technology, applied in the field of DPP-4-related diseases, dipeptidyl peptidase IV (DPP-4) inhibitor compounds, can solve the problem of GLP-1 inactivation and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073]
[0074] experiment procedure:
[0075] first step:
[0076] Method A
[0077] Weigh Boc-(R)-3-amino-4-(2,4,5-trifluorophenyl)butanoic acid (1.0g, 3.0mmol), anhydrous piperazine (0.26g, 3.0mmol), HBTU ( 2.27g, 6.0mmol) was added into a 100ml round bottom flask, dissolved in 20ml of DMF, then DIEA (0.78g, 6.0mmol) was added to the reaction solution, and stirred at room temperature for 1.5 hours. TLC detection (petroleum ether: ethyl acetate = 4:1), the reaction was completed, the reaction solution was added to 10 times the amount of water, a white solid was precipitated, filtered, the filter cake was washed with water, and the obtained solid was dried.
[0078] Method B
[0079] Weigh Boc-(R)-3-amino-4-(2,4,5-trifluorophenyl)butanoic acid (1.0g, 3.0mmol), anhydrous piperazine (0.26g, 3.0mmol), DCC ( 6.0mmol) and HoBt (6.0mmol) were added to a 100ml round bottom flask, dissolved with 20ml DMF, then DIEA (12.0mmol) was added to the reaction solution, and stirred at ...
Embodiment 2
[0085] Chemical reaction formula:
[0086]
[0087] Experimental process: The specific experimental process of compound 2 is similar to that of Example 1, refer to Example 1.
[0088] MSm / z (ESI):613.5[M+Na]
[0089] 1 H-NMR (500MHz, deuterated DMSO): δ7.31~7.38(m, 2H), 7.13~7.22(m, 2H), 4.01~4.15(m, 2H), 4.42(m,1H), 3.67(s ,3H), 2.80~2.91(m.4H), 2.59~2.72(m,4H).
Embodiment 3
[0091] Chemical reaction formula:
[0092]
[0093] Experimental process: The specific experimental process of compound 3 is similar to that of Example 1, refer to Example 1.
[0094] MSm / z(ESI):539.3[M+H]
[0095] 1 H-NMR (500MHz, deuterated DMSO): δ7.60~7.65(m, 4H), 7.31~7.38(m, 2H), 7.13~7.22(m, 2H), 4.42(m, 1H), 4.01~4.15 (m, 2H), , 3.67 (s, 3H), 2.80~2.91 (m.4H), 2.59~2.72 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com