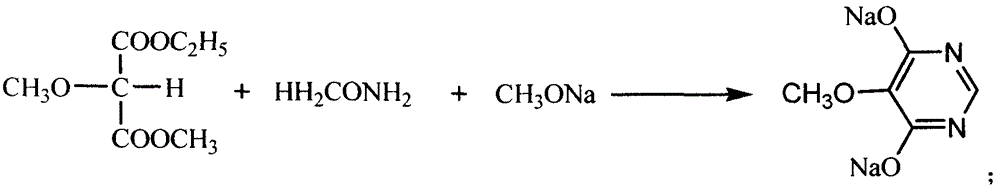Preparation method of 5-methoxy-4,6-dichloropyrimidine
A technology of sodium methoxypyrimidine and dichloropyrimidine, which is applied in the field of preparation of cyclic sulfonamide intermediates, can solve problems such as cross-temperature, incompatible with environmental protection, unfavorable reaction temperature, etc., and achieves reduction of production cost and personal safety. the effect of the threat
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
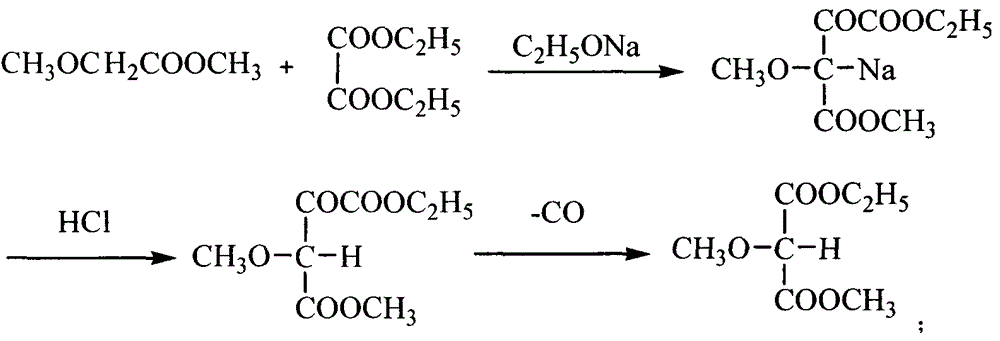
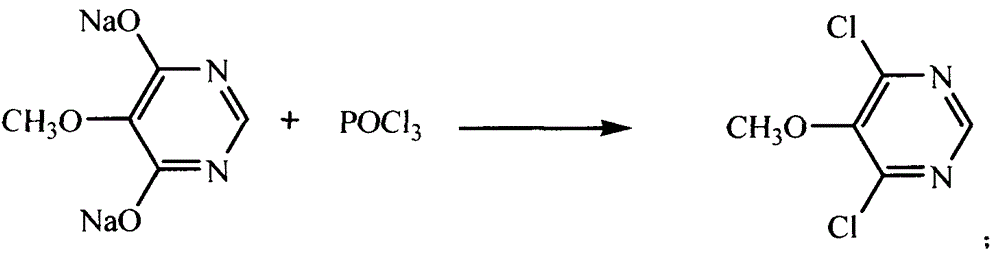
[0026] The preparation method of 5-methoxy-4,6-dichloropyrimidine of the present invention comprises the following steps: (a) adding phosphorus trichloride into the reaction vessel, and raising the temperature to 70-80°C, and adding to the reaction vessel within 0.5-1.5 hours Add 4,6-dihydroxy-5-methoxypyrimidine sodium, control the temperature at 80-95°C, and then reflux at 110-120°C for 2-6 hours to obtain a reaction mixture; (b) the The mixture is cooled to below 90°C, and an organic solvent is added to stir and dissolve, then poured into deionized water for hydrolysis, and left to separate to form the first water layer and the first organic solvent layer; (c) adding the first organic solvent to the adding lye to the layer to adjust the pH value to 6.5-7, and standing again to form a second water layer and a second organic solvent layer; (d) taking the second organic solvent layer and distilling off the organic solvent therein. On the one hand, the use of phosphorus trichlo...
Embodiment 1
[0030] This example provides a preparation method of 5-methoxy-4,6-dichloropyrimidine, and the specific feeding ratio is shown in Table 1:
[0031] Table 1 Reaction raw materials and their feeding ratio
[0032]
[0033] The specific operation process is as follows:
[0034] (a) Add 825kg of phosphorus trichloride into the dry chlorination reaction pot, turn on the reflux and hydrogen chloride gas absorption device, heat the steam to 70°C, turn off the steam and naturally raise the temperature to 80°C, and slowly add 4,6-dihydroxy- 5-Methoxypyrimidine sodium, control the addition speed so that the addition time is 0.5-1.5 hours, and the internal temperature is controlled at 80-95°C; heat the chlorination reaction pot, and reflux at 110-120°C for 4 hours;
[0035] (b) Distill under reduced pressure and reclaim phosphorus oxychloride (350kg) until the reaction residue is dry; close the steam valve, cool to below 90°C, add 900L trichlorethylene, stir and dissolve; put 1500Kg ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com