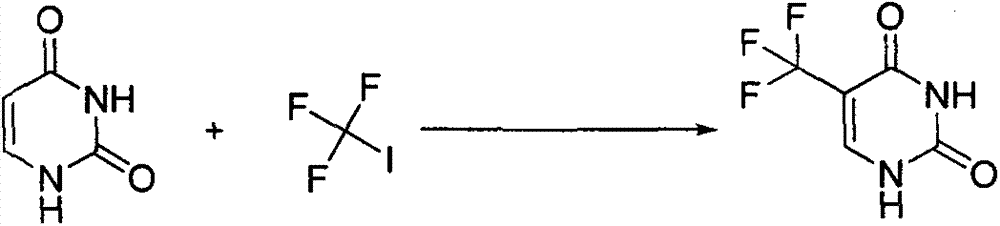
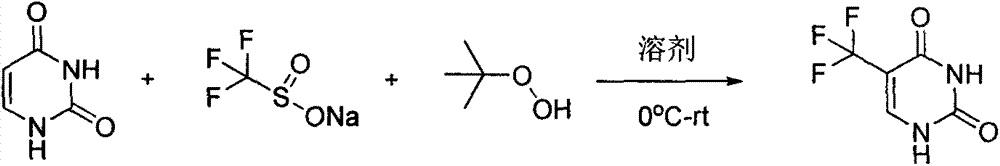
Synthesis method of 5-(trifluoromethyl) uracil
A technology of trifluoromethyluracil and uracil, which is applied in the field of preparation of 5-trimethyluracil, can solve the problems of high price, inconvenient purification, unsuitable for industrial operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] 1) Add 100 g (0.89 mol) of raw material uracil, 460.7 g (2.95 mol) of sodium trifluoromethanesulfinate and 5.0 L of water into the reaction flask, and cool in an ice bath.
[0012] 2) Slowly add 474 g (5.27 moles) of tert-butyl hydroperoxide dropwise, keeping the system temperature below 10°C. After the dropwise addition, the temperature was naturally raised to room temperature, 20 g of copper sulfate was added, and stirring was continued at 250 rpm.
[0013] 3) LCMS detects that the raw material reacts almost completely after 5 days
[0014] 4) Add saturated aqueous sodium thiosulfate solution to quench the peroxide, stir for 1 hour and then filter.
[0015] 5) The filtrate was extracted three times with 5.5 L of ethyl acetate, the organic phases were combined, washed with water (3 L) and saturated brine (3 L), dried over anhydrous sodium sulfate, filtered, and spin-dried to obtain a white solid.
[0016] 6) The white solid obtained in 5 was beaten with 550 mL (EtOAc...
Embodiment 2
[0018] 1) Add 100 g (0.89 mol) of raw material uracil, 460.7 g (2.95 mol) of sodium trifluoromethanesulfinate and 5.0 L of water into the reaction flask, and cool in an ice bath.
[0019] 2) Slowly add 474 g (5.27 moles) of tert-butyl hydroperoxide dropwise, keeping the system temperature below 10°C. After the dropwise addition, the temperature was naturally raised to room temperature, 20 g of copper sulfate was added, and stirring was continued at 500 rpm.
[0020] 3) LCMS detects that the raw material reacts almost completely after 5 days
[0021] 4) Add saturated aqueous sodium thiosulfate solution to quench the peroxide, stir for 1 hour and then filter.
[0022] 5) The filtrate was extracted three times with 5.5 L of ethyl acetate, the organic phases were combined, washed with water (3 L) and saturated brine (3 L), dried over anhydrous sodium sulfate, filtered, and spin-dried to obtain a white solid.
[0023] 6) The white solid obtained in 5 was beaten with 550 mL (EtOAc...
Embodiment 3
[0025] 1) Add 100 g (0.89 mol) of raw material uracil, 460.7 g (2.95 mol) of sodium trifluoromethanesulfinate and 5.0 L of water into the reaction flask, and cool in an ice bath.
[0026] 2) Slowly add 474 g (5.27 moles) of tert-butyl hydroperoxide dropwise, keeping the system temperature below 10°C. After the dropwise addition, the temperature was naturally raised to room temperature, and continued to stir at 250 per minute for 5 days.
[0027] 3) Add saturated aqueous sodium thiosulfate solution to quench the peroxide, stir for 1 hour and then filter.
[0028] 4) The filtrate was extracted three times with 5.5 L of ethyl acetate, the organic phases were combined, washed with water (3 L) and saturated brine (3 L), dried over anhydrous sodium sulfate, filtered, and spin-dried to obtain a white solid.
[0029] 5) The white solid obtained in 4 was beaten with 550 mL (EtOAc:PE=1:10) for 2 hours, filtered, the filter cake was washed with PE, and dried to obtain 75 g of the produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com