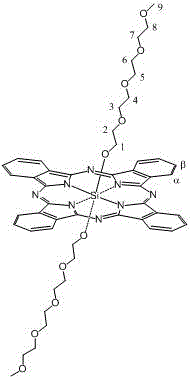
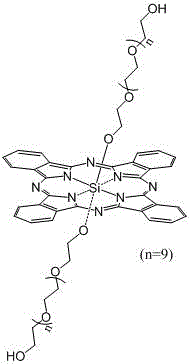
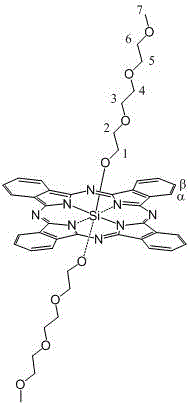
A kind of axial terminal hydroxyl substituted silicon phthalocyanine and its self-assembly
A silicon phthalocyanine and hydroxyl-terminated technology, which is applied in the field of axially-terminated hydroxyl-substituted silicon phthalocyanine and its self-assembly, can solve the problem that the application potential of nano-self-assembly has not yet been explored, the clinical application is limited, and the skin phototoxicity is high. problem, to achieve high photodynamic anticancer activity, high practical application effect, and clear structure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of silicon phthalocyanine substituted by axial terminal hydroxyl group of the present invention is: (1) using dichloro silicon phthalocyanine and triethylene glycol, tetraethylene glycol, PEG400 or PEG600 as reactants, and the molar ratio of the two is 1 :1~20, using toluene, xylene or dioxane as a solvent, under the protection of nitrogen, react at 100~130°C for 1~20 hours, and remove excess raw materials and impurities by solvent method and column chromatography, The resulting axial polyethylene glycol-modified silicon phthalocyanine. (2) Using dichlorosilicon phthalocyanine and p-hydroxyphenylpropionic acid as reactants, the molar ratio of the two is 1:1~20, using toluene, xylene or dioxane as solvent, under the protection of nitrogen , reacted at 100~130°C for 1~20 hours, separated and removed excess raw materials and impurities by solvent method and column chromatography, and obtained axial 3-(4-hydroxyphenyl)ethylcarbonyloxy and 4-(2- Carbo...
Embodiment 1
[0038] Synthesis of bis(2-(2-(2-hydroxyethoxy)ethoxy)ethoxy)silyl phthalocyanine
[0039] Under nitrogen protection, dichlorosilicon phthalocyanine (100 mg, 0.164 mmol), triethylene glycol (1.640~3.280 mmol, preferably 2.460 mmol) and NaH (0.01~0.02 mmol, preferably 0.016 mmol) were added to toluene 7~ 15 ml (preferably 10 ml), reflux for 12-24 hours (preferably 14 hours). The solvent was removed by vacuum rotary evaporation, and washed with water to obtain a blue crude product. The crude product was purified by a silica gel column, using ethyl acetate: chloroform (5:1) as the eluent, and the second fraction was collected, concentrated and dried to obtain a blue product with a yield of 32.91%. The maximum absorption peak of the product in DMF is located at 673 nm, and the maximum absorption wavelength in 1% castor oil derivative (Cremophor EL, wt%) aqueous solution is located at 676 nm.
[0040] The structure of the product is shown in the following formula, and the characte...
Embodiment 2
[0045] Synthesis of bis(2-(2-(2-methoxyethoxy)ethoxy)ethoxy)silyl phthalocyanine
[0046] Under nitrogen protection, dichlorosilicon phthalocyanine (100 mg, 0.164 mmol), triethylene glycol monomethyl ether (1.640~3.280 mmol, preferably 2.460 mmol) and NaH (0.01~0.02 mmol, preferably 0.016 mmol) were added to In 7~15 ml of toluene (preferably 10 ml), reflux for 12~24 hours (preferably 12 hours). The solvent was removed by vacuum rotary evaporation, and washed with water to obtain a blue crude product. The crude product was purified by silica gel column, using ethyl acetate solvent as eluent, and the second component was collected, concentrated and dried to obtain a blue product with a yield of 32.91%. The maximum absorption peak of the product in DMF is located at 674 nm, and the maximum absorption wavelength in 1% castor oil derivative (Cremophor EL, wt%) aqueous solution is located at 677 nm.
[0047] The structure of the product is shown in the following formula, and the c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com