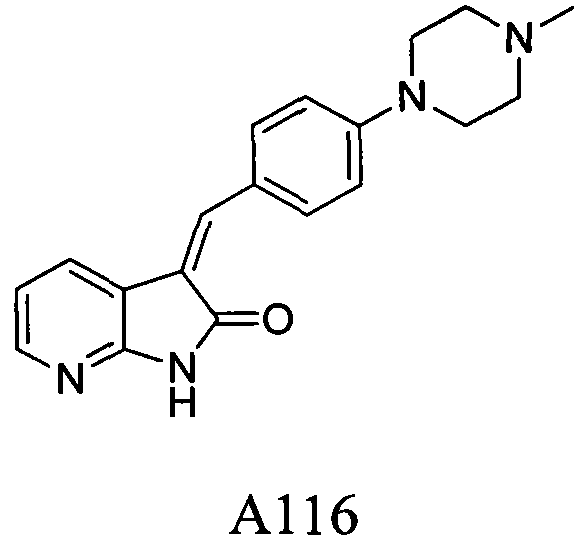
A novel azaindole-2-one FGFR1 inhibitor and its antitumor activity
A tumor, A116 technology, applied in the chemical structure of azaindol-2-one compounds, anti-tumor applications, can solve the problems of large toxic side effects, inhibitory effects, multi-drug resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 compound inhibits the activity and selectivity of FGFR1 in vitro
[0021] In vitro activity screening of FGFR1 kinase: The method used in the experiment is Caliper Mobility Shift Assay, which is a detection platform based on the mobility detection technology of microfluidic chip technology. Experimental steps: configure 1.25x kinase reaction buffer (62.5mmol / L HEPES, pH7.5; 0.001875% Brij-35; 12.5mmol / LMgCl2; 2.5mM DTT) and kinase reaction termination solution (100mmol / L HEPES, pH7.5 ; 0.015% Brij-35; 0.2% Coating Reagent#3); Add 10 μl of 2.5x FGFR1 kinase solution (in 1.25x kinase reaction buffer add kinase in solution), incubate at room temperature for 10min, then add 10μl of 2.5x substrate peptide solution (add FAM-labeled peptide and ATP in 1.25x kinase reaction buffer), add 25μl kinase reaction after reacting at 28℃ for a specific time stop solution. Test and collect data on Caliper, inhibition rate of kinase activity=(max-conversion) / (max-min)*100. "m...
Embodiment 2
[0024] Antitumor activity verification of compound on the cell level in Example 2
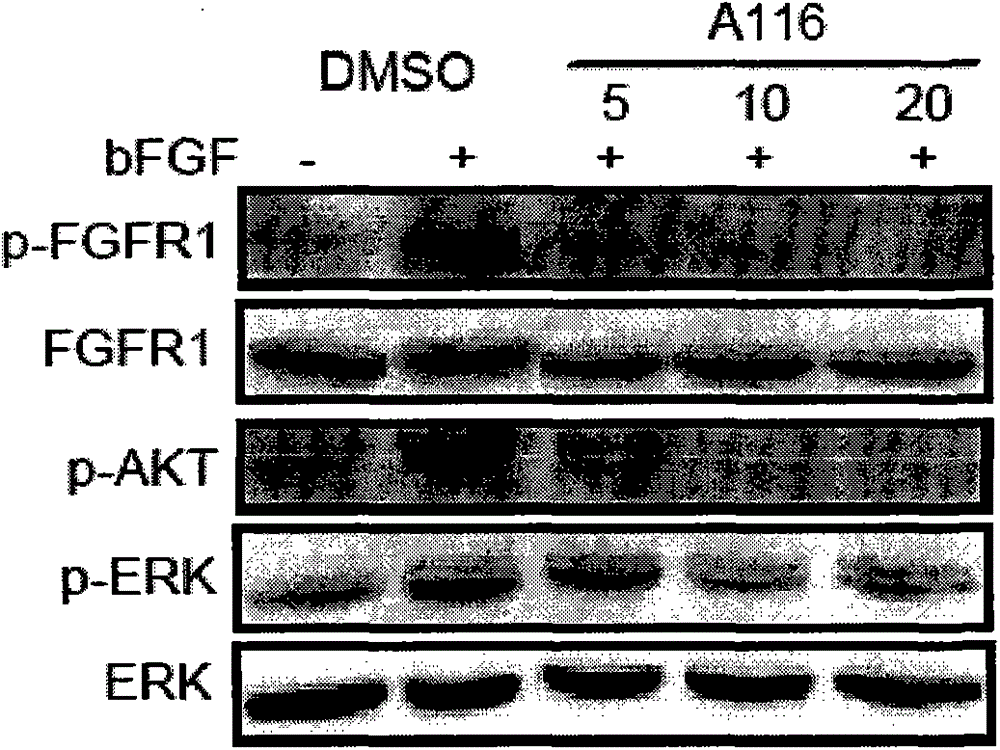
[0025] The compound A116 was formulated into 5, 10 and 20um concentration solutions with DMSO respectively, and the H460 and MRC-5 cells with high expression of FGFR1 were treated with bFGF and bFGF combined with the above-mentioned active compounds at different concentrations, and the anti-tumor proliferation activity of the two compounds was detected At the same time, total cell protein was extracted, and Western blotting was used to detect the changes in the phosphorylation levels of pro-proliferation signal molecules FGFR, ERK and AKT in the bFGF / FGFR signaling pathway in cells. Compound A116 has good anti-tumor proliferation activity, and can strongly inhibit p-FGFR1, p-AKT and p-ERK levels at three concentrations of 5, 10 and 20um (see image 3 ).
Embodiment 3
[0026] Example 3 Non-ATP Competitive Inhibitory Binding Mode of Compound A116 to FGFR1
[0027] Compound A116 was selected, and 10 concentration gradients were configured from 0 to 100 μmol / L using the kinase inhibitory activity screening method, and each concentration was tested in 8 different ATP concentration reaction systems for the conversion of substrate peptides catalyzed by FGFR1 kinase. Influence, the competitive or non-competitive inhibition curves were fitted by software such as GraphPad Prism, and non-ATP competitive enzyme inhibition reaction parameters such as Michaelis constant (Km) and enzymatic maximum reaction rate (Vmax) were calculated at each concentration. Experimental results such as Figure 4 As shown, compared with the standard pd173074, as the concentration of ATP increases, the combination of compound A116 and FGFR1 is not affected by the concentration of ATP, indicating that the inhibitory binding mode of compound A116 and FGFR1 is non-ATP competiti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com