A method for removing residual hydrogen fluoride in fluosilicic acid
A technology of fluorosilicic acid and hydrofluoric acid, applied in the direction of halogenated silicon compounds, etc., can solve the problem of difficult disposal of waste FRP materials, and achieve the effects of reducing raw material cost, reducing pollution, and inhibiting hydrolysis reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
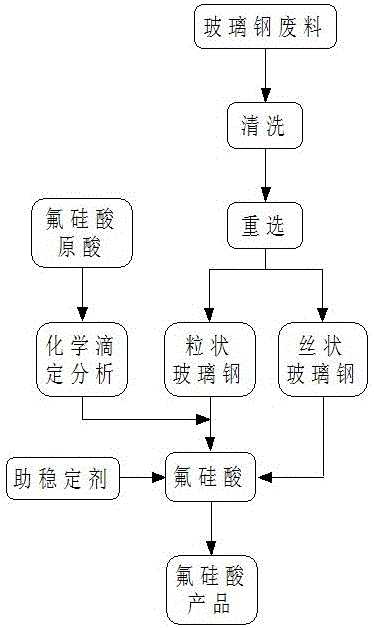
[0028] A method for producing fluosilicic acid by utilizing glass fiber reinforced plastic waste slag, comprising the following specific steps:
[0029] 1) Take FRP scraps and incomplete waste;
[0030] 2) After washing and drying the above-mentioned glass fiber reinforced plastic scraps and incomplete wastes, they are ground and re-selected to obtain silk flocculent glass fiber reinforced plastics and granular glass fiber reinforced plastics;
[0031] 3) Analysis and determination of the content of fluosilicic acid and hydrofluoric acid in industrial fluosilicic acid;
[0032] 4) Add granular glass fiber reinforced plastic to industrial fluosilicic acid and stir for reaction. The amount of granular glass fiber reinforced plastic is 100% of the weight ratio of hydrofluoric acid contained in industrial fluosilicic acid; the reaction temperature is 20°C, and the reaction time is controlled At 72h, the stirring speed was 300 rpm;
[0033] 5) Filter the reaction solution to obta...
Embodiment 2
[0036] A method for producing fluosilicic acid by utilizing glass fiber reinforced plastic waste slag, comprising the following specific steps:
[0037] 1) Take FRP scraps and incomplete waste;
[0038] 2) Washing and drying the above-mentioned glass fiber reinforced plastic scraps and incomplete wastes, then grinding and re-selecting to obtain fibrous flocculent glass fiber reinforced plastics and granular glass fiber reinforced plastics;
[0039] 3) Analysis and determination of the content of fluosilicic acid and hydrofluoric acid in industrial fluosilicic acid;
[0040] 4) Add granular glass fiber reinforced plastic to industrial fluosilicic acid and stir for reaction. The amount of granular glass fiber reinforced plastic is 500% of the weight ratio of hydrofluoric acid contained in industrial fluosilicic acid; the reaction temperature is 45°C, and the reaction time is controlled At 0.5h, the stirring speed was 30 rpm;
[0041] 5) Filter the reaction solution to obtain f...
Embodiment 3
[0044] A method for producing fluosilicic acid by utilizing glass fiber reinforced plastic waste slag, comprising the following specific steps:
[0045] 1) Take FRP scraps and incomplete waste;
[0046] 2) After washing and drying the above-mentioned glass fiber reinforced plastic scraps and incomplete wastes, and then grinding and re-selecting, silk flocculent fiberglass and granular fiberglass are obtained;
[0047] 3) Analysis and determination of the content of fluosilicic acid and hydrofluoric acid in industrial fluosilicic acid;
[0048] 4) Add granular glass fiber reinforced plastic to industrial fluosilicic acid and stir for reaction. The amount of granular glass fiber reinforced plastic is 300% of the weight ratio of hydrofluoric acid contained in industrial fluosilicic acid; the reaction temperature is 37°C, and the reaction time is controlled At 2.5h, the stirring speed was 120 rpm;
[0049] 5) Filter the reaction solution to obtain fluosilicic acid products and g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com