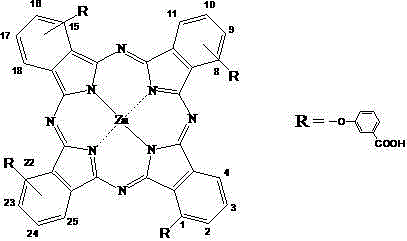
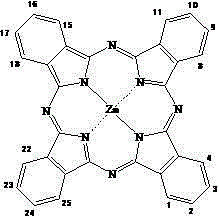
A compound of phycocyanin and phthalocyanine and its preparation method and application
A technology of phycocyanin and compound, which is applied in the direction of botanical equipment and methods, applications, medical preparations containing active ingredients, etc., can solve the problems of easy aggregation of zinc, increased biological activity, poor water solubility, etc., and overcome hydrophobicity , good water solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The preparation method of the complex of phycocyanin and phthalocyanine according to the present invention comprises the following main steps: (a) dissolving the phthalocyanine complex in a suitable solvent, preparing a certain concentration of the phthalocyanine complex solution, and dissolving Water-soluble phycocyanin is dissolved in buffer solution or buffer solution containing urea to prepare a certain concentration of water-soluble phycocyanin solution; (b) According to a certain feeding ratio, gradually add the phthalocyanine complex solution to the water-soluble phycocyanin solution to form a reaction mixture; (c) shake or stir the above reaction mixture at 25-37°C for 2-12 hours, so that the phthalocyanine complex and water-soluble phycocyanin Combined into a complex to obtain a mixed solution containing the complex and free phthalocyanine complexes; (d) separate the above solution by gel permeation chromatography with a buffer solution as an eluent; (e) separat...
Embodiment 1
[0040] Extraction and Purification of Phycocyanin
[0041] (1) Crude extraction of phycocyanin
[0042] Prepare a phosphate buffer solution with pH=7, weigh 10g of spirulina powder, dissolve it in 250mL of buffer solution, oscillate ultrasonically for 5 minutes, and place it in a -20°C refrigerator for 12 hours. Its electronic absorption spectrum.
[0043] (2) Salting out of phycocyanin
[0044] Take the phycocyanin water extract, add saturated ammonium sulfate solution or ground ammonium sulfate powder, make the saturation of ammonium sulfate in the solution respectively 40%, 4°C overnight, carry out salting out, centrifuge at 12000r / min for 20min, and put the above Dilute the supernatant to an appropriate multiple or dissolve the precipitate with an appropriate amount of distilled water, then measure the UV-visible absorption spectrum, and measure the wavelength range of 250-800nm to calculate the purity and concentration of the protein in the solution.
[0045] (3) Desal...
Embodiment 2
[0051] Synthesis of 1,8(11), 15(18), 22(25)-tetrakis(3-carboxyphenoxy)zinc phthalocyanine
[0052] (1) Preparation of intermediate 3-(3-carboxyphenoxy)phthalonitrile:
[0053] Add 0.69g (5mmol) 3-hydroxybenzoic acid and 0.69-2.08g (4-6mmol) (preferably 0.87g, 5mmol) 3-nitrophthalonitrile into 35ml DMF, blow nitrogen, stir at room temperature, after 10 minutes Add 2.1g (15mmol) of potassium carbonate and react for 24 hours. Suction filter the reaction mixture, collect the filtrate, add 200ml ice-water mixture to the filtrate, adjust the pH value to 1~3 (preferably 2) with 2mol / L HCl solution, the product precipitates out, filter, wash with water until neutral, collect and filter The cake was dried under normal pressure at 70°C to obtain a crude product. The crude product was dissolved with a small amount of DMF, then a large amount of water was added to precipitate a precipitate, filtered, washed with water three times, and dried under normal pressure at 70°C to obtain 1.11 g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com