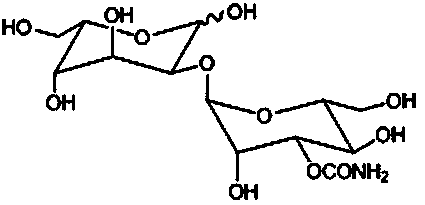
Method for synthesizing 1,3,4,6-tetraacetyl-L-gulose
A technology of tetraacetyl and gulose, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of high requirements for experimental operators, difficult to scale up production, long synthetic routes, etc., and achieve the synthetic route. Short, easy-to-operate, easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
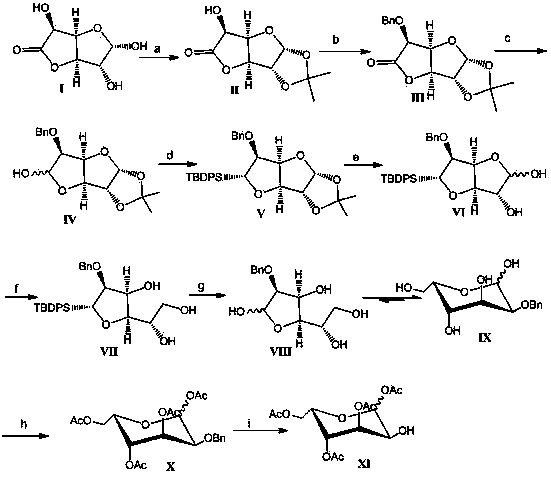
Examples
Embodiment 1
[0043] 1.1 Preparation of compound Ⅱ
[0044] Dissolve 1.5g of compound I in 15ml of acetone at room temperature, add 1.25ml of concentrated sulfuric acid dropwise after cooling in an ice bath, and stir the reaction mixture at room temperature for 1-2 hours. Add NaHCO to the reaction solution 3 50g of powder, until pH ≥ 7, filtered out the solid, spin-dried the solvent, and crystallized with ethyl acetate to obtain a white solid, namely compound II, 1.56g, yield 85%.
[0045] [a] D 25 +55.3 (c 0.92 CHCl3).
[0046] 1 H NMR (400 MHz, CDCl 3 ) δ 5.99 (d, J = 3.5 Hz, 1H), 4.95 (dd, 4.2, 3.5, 1H), 4.83-4.82 (m, 2H), 4.50 (dd, J = 9.0, 4.2 Hz, 1H), 2.86 (d, J = 9.0 Hz, 1H), 1.53 (s, 3H), 1.36 (s, 3H).
[0047] MS (EI): m / e = 216.
[0048] 1.2 Preparation of compound Ⅱ
[0049] Dissolve 1.5g of compound I in 15ml of acetone at room temperature, add 1.25ml of concentrated sulfuric acid dropwise under ice-bath cooling, and warm the reaction mixture to room temperature an...
Embodiment 2
[0059] 2.1 Preparation of Compound III
[0060] 10.7 g of compound II was dissolved in 50 ml of ethyl acetate, 7 g of silver oxide and 7 ml of benzyl bromide were added at room temperature, and the reaction mixture was stirred overnight at room temperature. The solid in the reaction mixture was filtered off, and the filtrate was concentrated and purified by column chromatography to obtain an oily substance, namely compound III, 13 g, with a yield of 86%.
[0061] [a] D 26 +44.5 (c 0.92, CHCl3).
[0062] 1 H NMR (400 MHz, CDCl 3 ) δ 7.37 (m, 5H), 6.04 (d, J = 3.6 Hz, 1H), 4.92 (s, 2H), 4.86 (dd, J = 4.3, 2.9HZ, 1H), 4.79 (d, J = 3.6 Hz, 1H), 4.71 (d, J = 2.9 Hz, 1H), 4.25 (d, J = 4.3 Hz, 1H), 1.50 (s, 3H), 1.34 (s, 3H).
[0063] MS (EI): m / e=306.
[0064] 2.2 Preparation of Compound III
[0065] 10.7g of compound II was dissolved in 50ml of DMF, 7g of silver oxide and 7ml of benzyl bromide were added at room temperature, and the reaction mixture was stirred at...
Embodiment 3
[0075] 3.1 Preparation of mixture IV
[0076] 1g compound III was dissolved in 8ml CH 2 Cl 2 Cool to -78°C, and slowly add 7.4ml of 1.1M DIBAL-H dropwise to the reaction solution. The reaction mixture was stirred at -40°C for 2 hours, 15ml of saturated sodium potassium tartrate solution was added to the reaction solution, stirred overnight at room temperature, allowed to stand, and separated into layers, the organic phase was separated, dried and purified by column chromatography to obtain an oily mixture IV (poor To isomers) 0.9g, yield 90%, the mixture was directly used in the next reaction.
[0077] 3.2 Preparation of mixture IV
[0078] 1g compound III was dissolved in 8ml CH 2 Cl 2 Cool to -78°C, slowly add 6.8ml of 1.1M DIBAL-H dropwise to the reaction solution. The reaction mixture was stirred at -40°C for 2 hours, 15ml of saturated sodium potassium tartrate solution was added to the reaction solution, stirred overnight at room temperature, allowed to stand, separ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com