Novel treatments
A therapy, technology for muscular dystrophy in the field of intracellular protein degradation inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0144] Summary
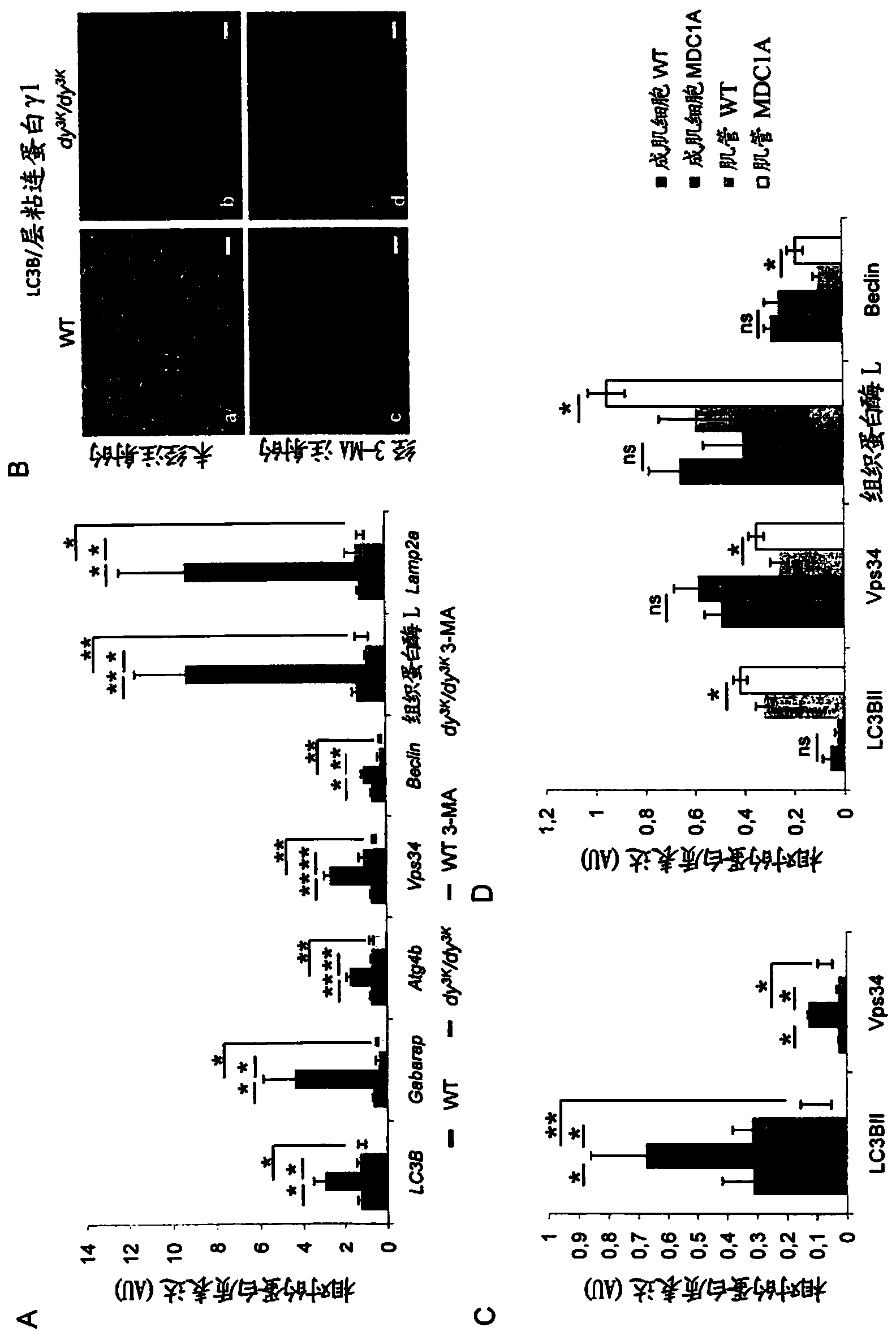
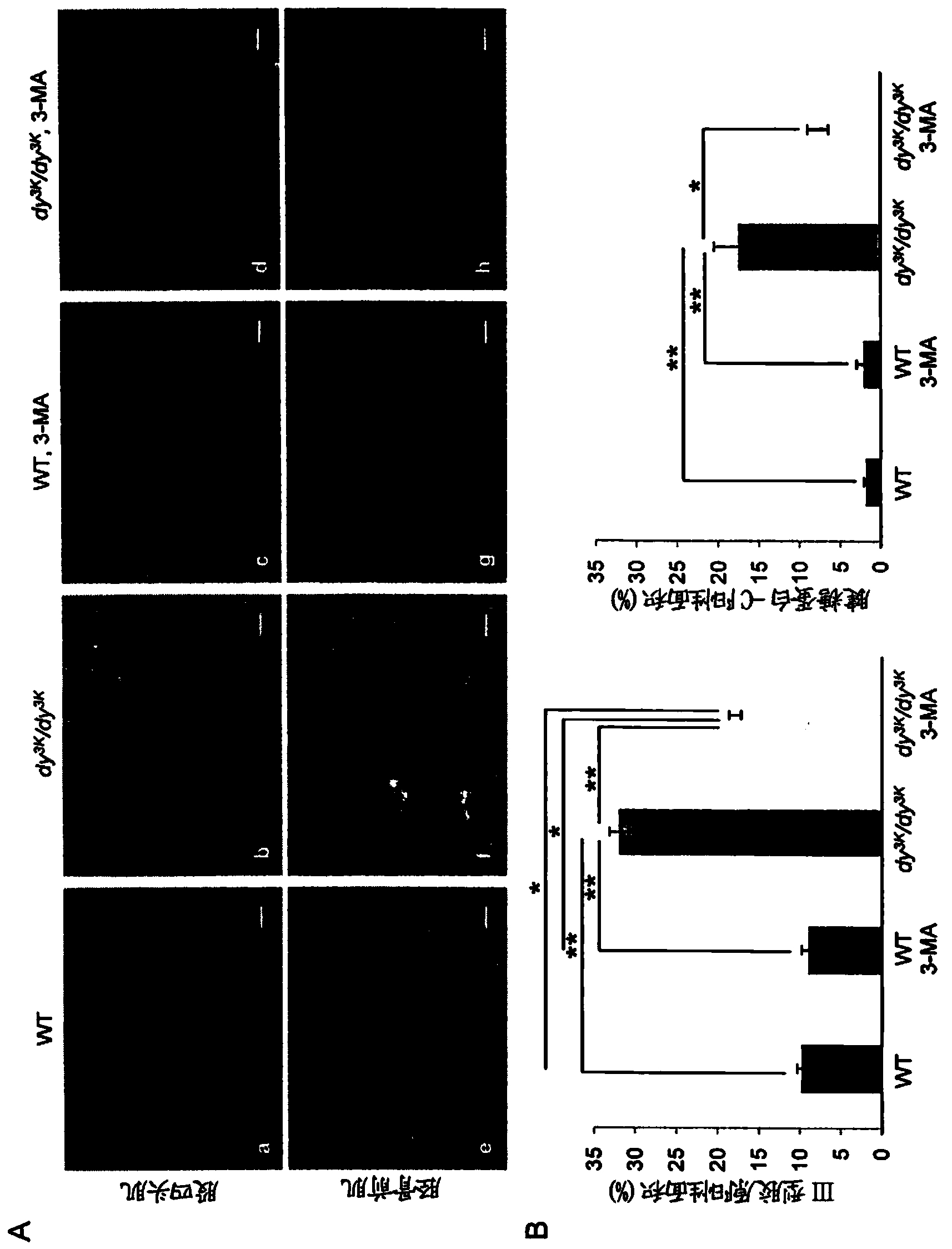
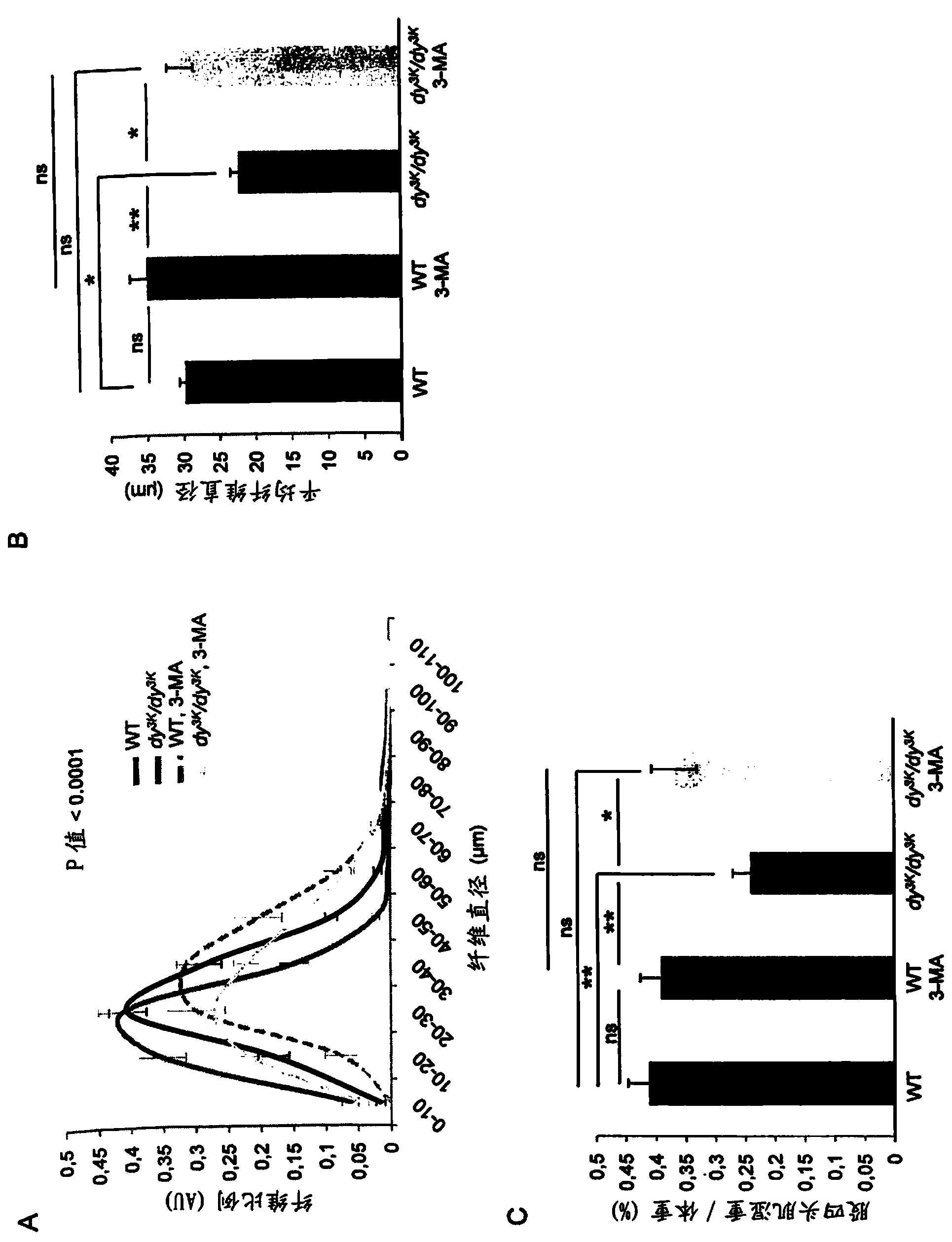
[0145] Laminin-α2 chain-deficient congenital muscular dystrophy (also known as MDC1A) is a severe and disabling disease. Recent studies have shown that increased proteasome activity is a hallmark of this disease. The autophagy-lysosomal pathway is another major system involved in the degradation of proteins and organelles in muscle cells. However, whether the autophagy-lysosomal pathway is overactive in muscular dystrophies, including MDC1A, remains to be determined. Studies using a laminin-α2 chain-deficient dy3K / dy3K mouse model and MDC1A patient muscle cells showed that autophagy-related gene expression was upregulated in laminin-α2-chain-deficient muscles. Furthermore, we found that autophagy inhibition significantly improved the dystrophic dy3K / dy3K phenotype. Specifically, it was shown that systemic injection of 3-methyladenine (3-MA) reduces muscle fibrosis, atrophy, apoptosis and increases muscle regeneration and weight. Importantly, longevity ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com