Application of salidroside in preparing medicine for treating duchenne muscular dystrophy
A technology of Duchenne muscular nutrition and salidroside, which is applied in drug combinations, pharmaceutical formulas, muscular system diseases, etc., and can solve problems such as no salidroside
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
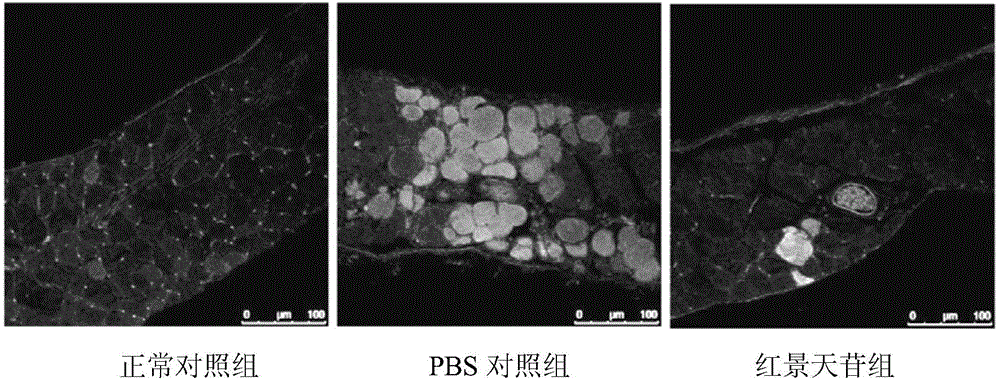
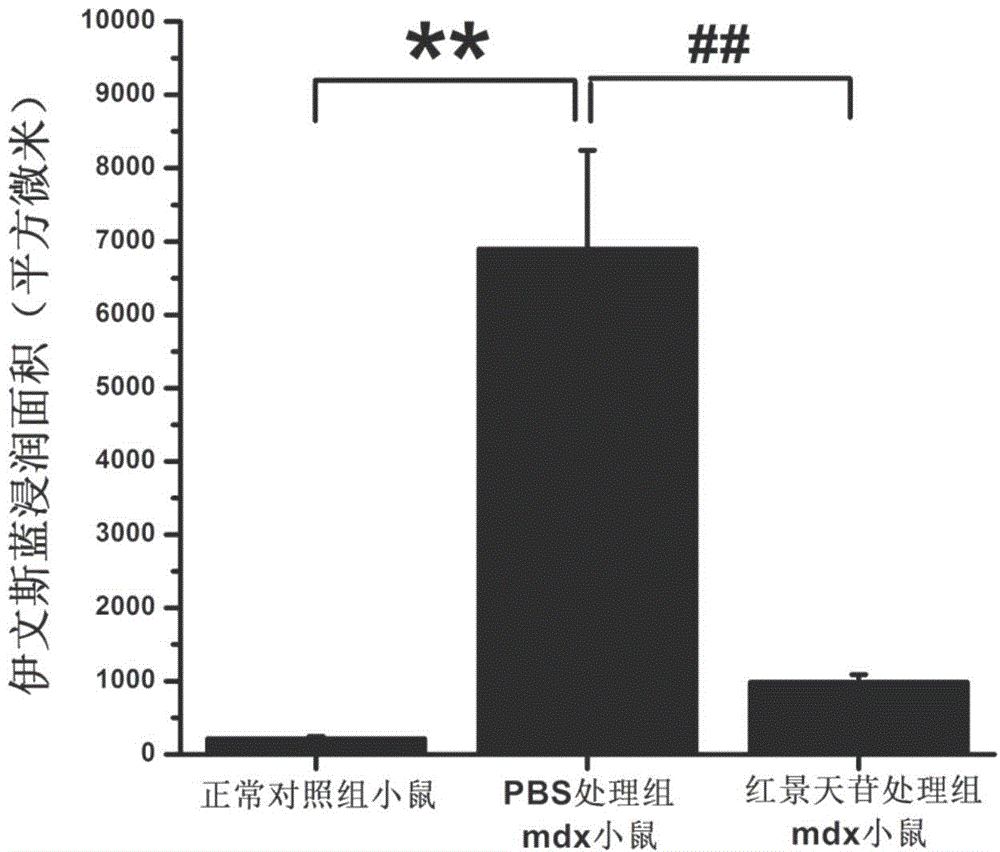
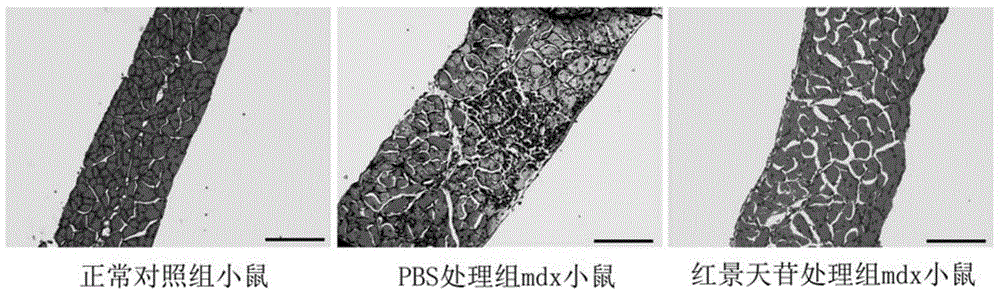
[0037] The sources of experimental animals and materials used in the following experiments are as follows:
[0038] Experimental animals: female mdx and its normal wild-type control C57BL / 10 mice were purchased from the Institute of Model Animals, Nanjing University, production license number: SCXK (Su) 2010-0001. mdx is a dystrophin gene knockout mouse, which is currently internationally recognized and the most widely used animal model for studying human Duchenne muscular dystrophy.
[0039] Grouping and administration of experimental animals:
[0040]In the following six experiments, the experimental animals of each experiment were divided into three groups, 6 in each group:
[0041] Salidroside treatment group: mdx mice were given 28 days of continuous intragastric administration, with a dose of salidroside 50 mg / d / kg;
[0042] PBS control treatment group: mdx mice were continuously gavaged with equal volume solvent PBS every day for 28 days;
[0043] Normal control grou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com