Analysis and evaluation method for releasing process of mixture chemome
A technology of analysis, evaluation and release process, applied in special data processing applications, instruments, electrical digital data processing, etc., can solve problems such as low accuracy and work efficiency, no direct standard, complex material basis, etc., to achieve composition Reliable principle, fast analysis and evaluation speed, and simple system structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
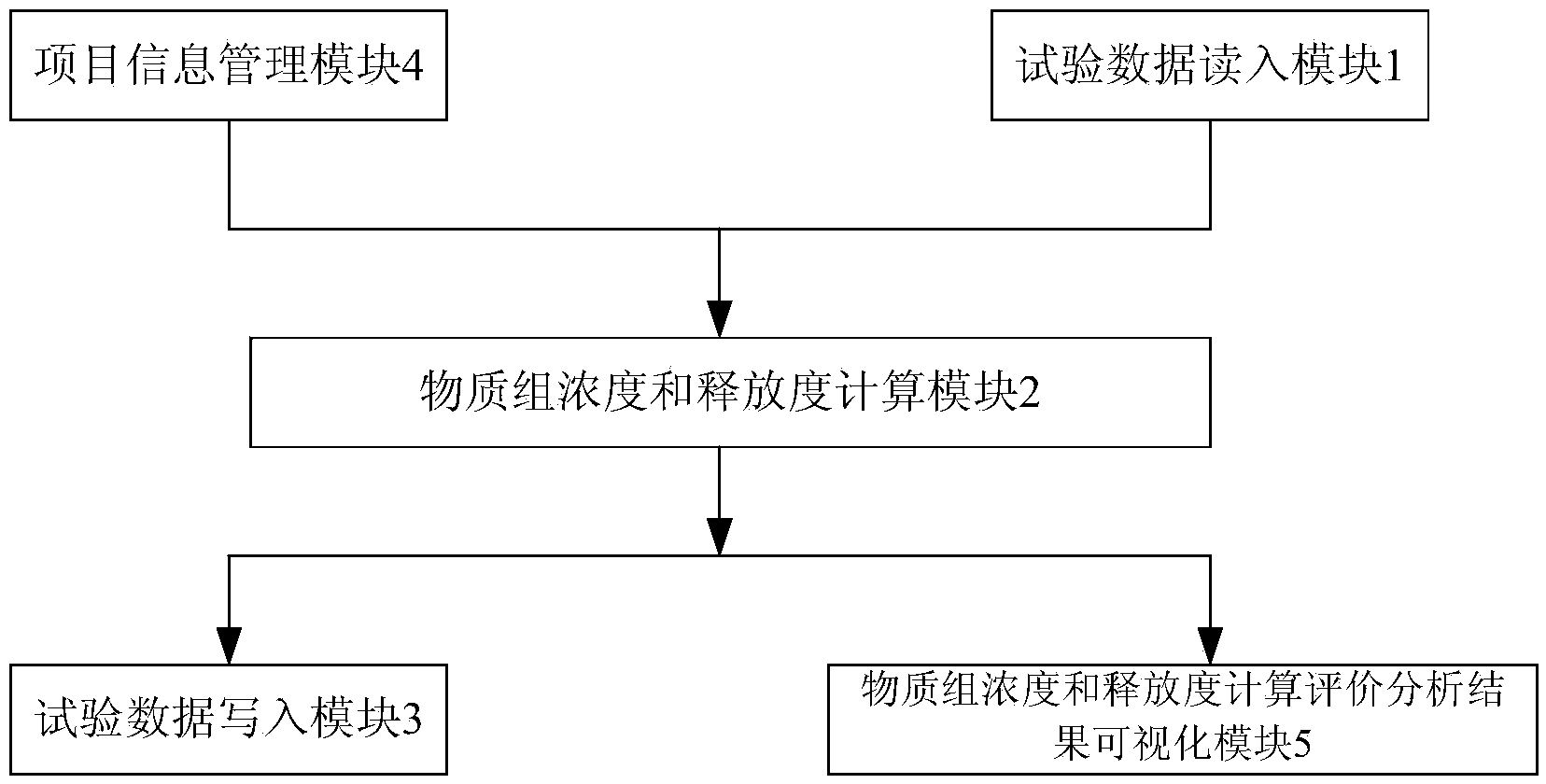
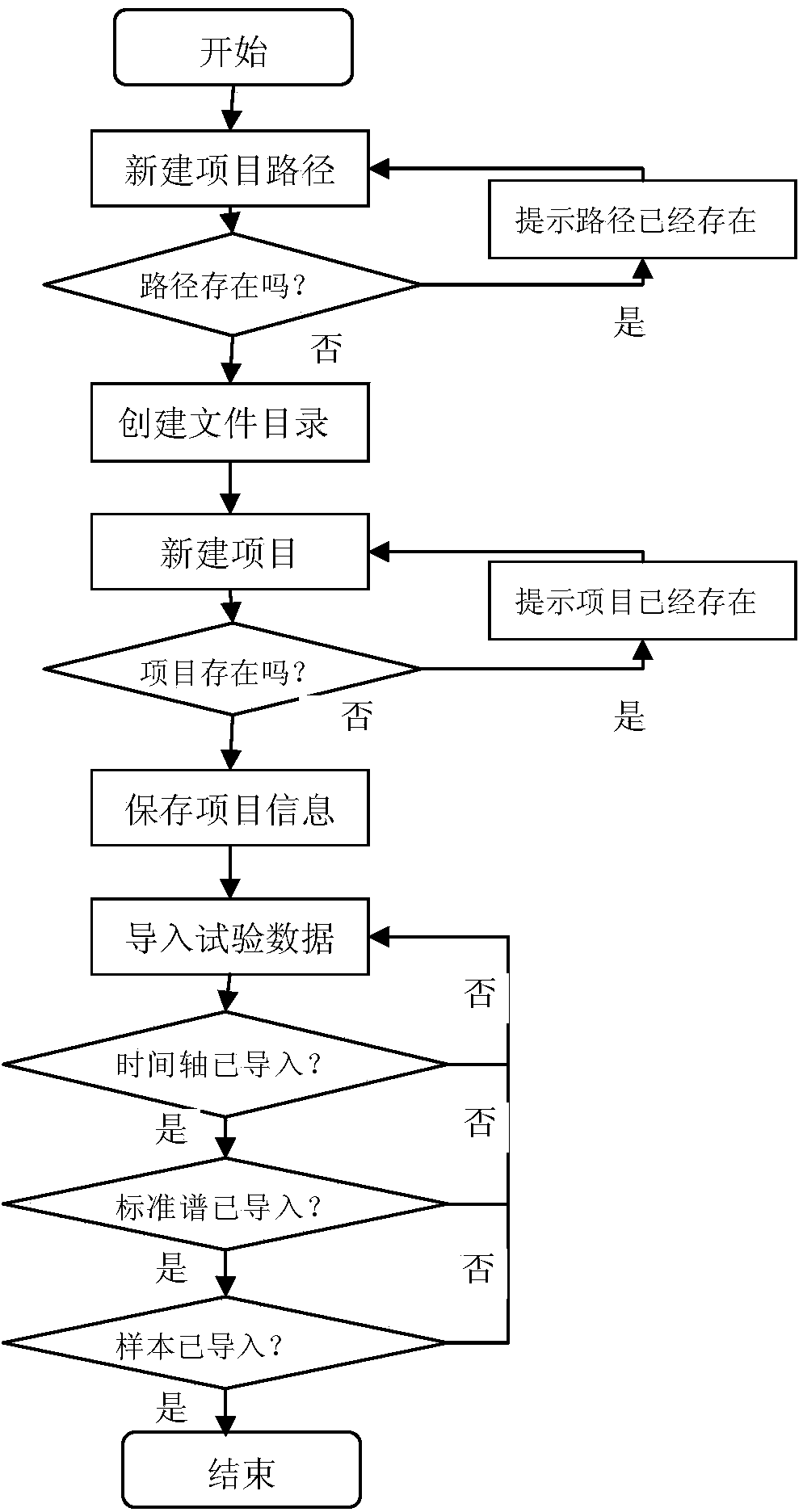
[0034] The main structure of the hardware composition of the computer analysis and evaluation system involved in this embodiment consists of a test data reading module 1, a substance group concentration and release calculation module 2, a test data writing module 3, a project information management module 4, and a substance group concentration and release Degree calculation evaluation analysis result visualization module 5 electrical information connection combination composition; project information management module 4 manages the project storage path, stores project information and project input and output files, realizes project information management, project information initialization and project information display operations, project information Class management uses object-oriented thinking to abstract project information categories based on project characteristics. The attributes of project information categories include project storage path, project name, number of samp...
Embodiment 2
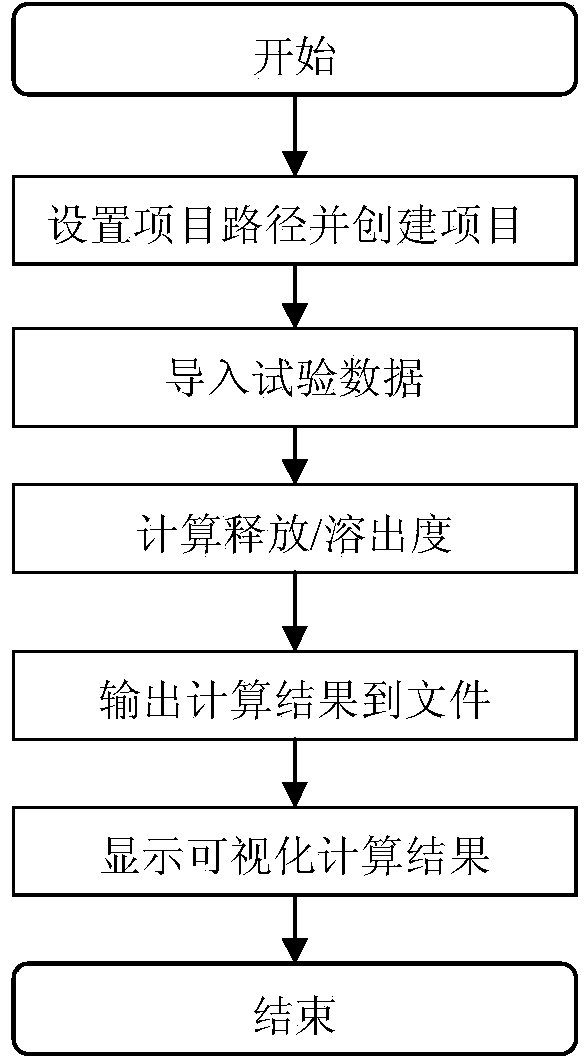
[0054] This embodiment provides a computer analysis and evaluation system and method for the release process of multi-component substance groups of pharmaceutical preparations. The independent client mode is adopted to realize the analysis of the sample data of the pharmaceutical compound preparations, and the number of pharmaceutical compound preparations based on the Kalman filtering method is provided. The calculation result of the cumulative release of the component substance group, and output the result to a file for storage, and can also display the visual result; the operating platform of the system must be compatible with a PC with a dual-core microprocessor or higher and a memory of 2G or more (1G) It runs on the machine, the server's operating system is Windows XP and above, and the necessary software for the system is Framework2.0. It is realized by object-oriented programming language and object-oriented development tool programming. The system supports different proj...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com