Double-schiff-base based on naphthol, synthesis thereof and application thereof as fluorinion sensor molecular
A Schiff base and fluoride ion technology, applied in the field of anion detection, can solve the problems of low sensitivity, quenching response, complex synthesis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0040] The preparation of the sensor molecule and the fluoride ion detection test paper of the present invention and the application in the detection of fluoride ion in drinking water will be further described below through specific examples.
[0041] 1. Preparation of sensor molecules
[0042] Add 10.0 mmol of 2-hydroxy-1-naphthaldehyde, 5.0 mmol of 1,4-butanediamine, 30 mL of absolute ethanol, and glacial acetic acid (0.5-1 mol) into a 50 mL reaction flask, and stir at reflux (85°C) for 24 hours. Cool and filter with suction to obtain a yellow solid, which is recrystallized from chloroform-ethanol to obtain the product.
[0043] Yield: 83%. m.p. 245-247℃, 1 H-NMR (d 6 -DMSO, 400 MHz) δ 12.2(s, 2H, -OH), 9.13 (s, 2H, =CH), 8.07~6.71 (m, 12H, -ArH), 3.73(m, 4H, =N-CH2) , 1.87~1.78 (m, 4H, -CH2). IR (KBr, cm -1 ) v: 3426(-OH), 3050(Ar-H), 1634(CH=N). MS: m / z: 395.57 [C 26 h 22 N 2 o 4 + H+]; Calcd for C 26 h 22 N 2 o 4 : 396.48.
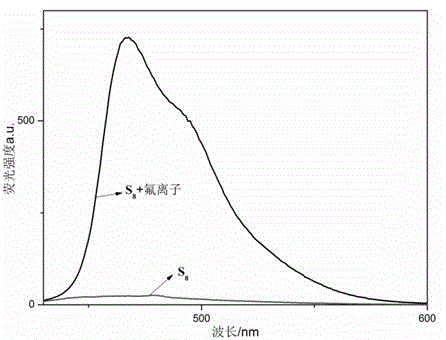
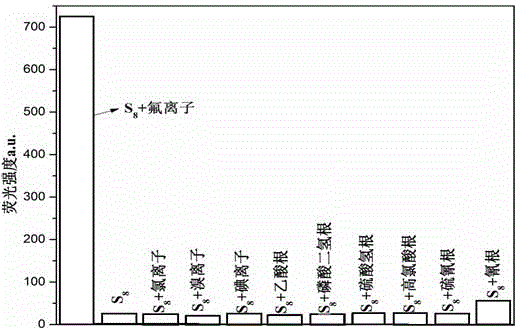
[0044] 2. Preparation of fluorid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com