Reactive organic metal hydroxide flame retardant and its preparation method
A technology of hydroxides and organic metals, applied in the treatment of dyed low-molecular organic compounds, fibrous fillers, etc., can solve the problems of low flame retardant efficiency, poor interface bonding, poor compatibility, etc., and increase the interface contact area , good mechanical properties, and the effect of improving flame retardant efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Take 50 parts of terephthalic acid and add it to 30 parts of N, N-dimethylformamide, ultrasonically oscillate for 15 minutes to obtain a terephthalic acid solution, and take another 17 parts of magnesium hydroxide, add it to the above solution, and use 150r / Min and stirred for 10 min, the suspension was heated to 90°C and stirred for 6 hours, then the suspension was filtered with suction, and the obtained solid was washed with 50 parts of dichloromethane for several times, and then dried at 60°C for 24 hours to obtain a Reactive organic magnesium hydroxide flame retardant.
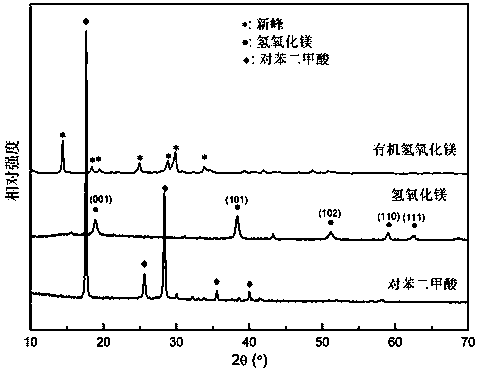
[0034] In the above experimental process, the infrared spectra of the prepared organometallic magnesium hydroxide (OMS), magnesium hydroxide (MH) and terephthalic acid (PTA) are as follows figure 1 As shown, in the spectrum of PTA, 1725cm -1 The stretching vibration absorption peak of C=O, and the stretching vibration absorption peak of -COO- at 1650cm-1, due to the strong association of hydroxyl ...
Embodiment 2
[0036]Take 100 parts of terephthalic acid and add it to 70 parts of N, N-diethylformamide, ultrasonically oscillate for 20 minutes to obtain a terephthalic acid solution, and take another 17 parts of magnesium hydroxide, add it to the above solution, and use 200r / Stir for 30 minutes, raise the temperature of the suspension to 110°C and stir for 5 hours, then filter the suspension with suction, wash the obtained solid with 100 parts of ethanol for several times, and then dry it at 100°C for 12 hours to obtain a reaction type Organic magnesium hydroxide flame retardant.
Embodiment 3
[0038] Take 50 parts of terephthalic acid and add it to 60 parts of N-methylpyrrolidone, and ultrasonically vibrate for 30 minutes to obtain a terephthalic acid solution. Take another 35 parts of magnesium hydroxide, add it to the above solution, and stir at 300 r / min for 30 minutes. The suspension was heated to 130°C and stirred thoroughly for 4 hours, then the suspension was filtered with suction, the obtained solid was washed several times with 90 parts of dichloromethane, and then dried at 80°C for 18 hours to obtain a reactive organic hydrogen Magnesium oxide flame retardant.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com