Determination method of 3-alkylated adenine DNA adducts in urine
A technology for DNA adducts and alkylated adenine, which is used in measuring devices, instruments, scientific instruments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0054] 1. Instruments and reagents:
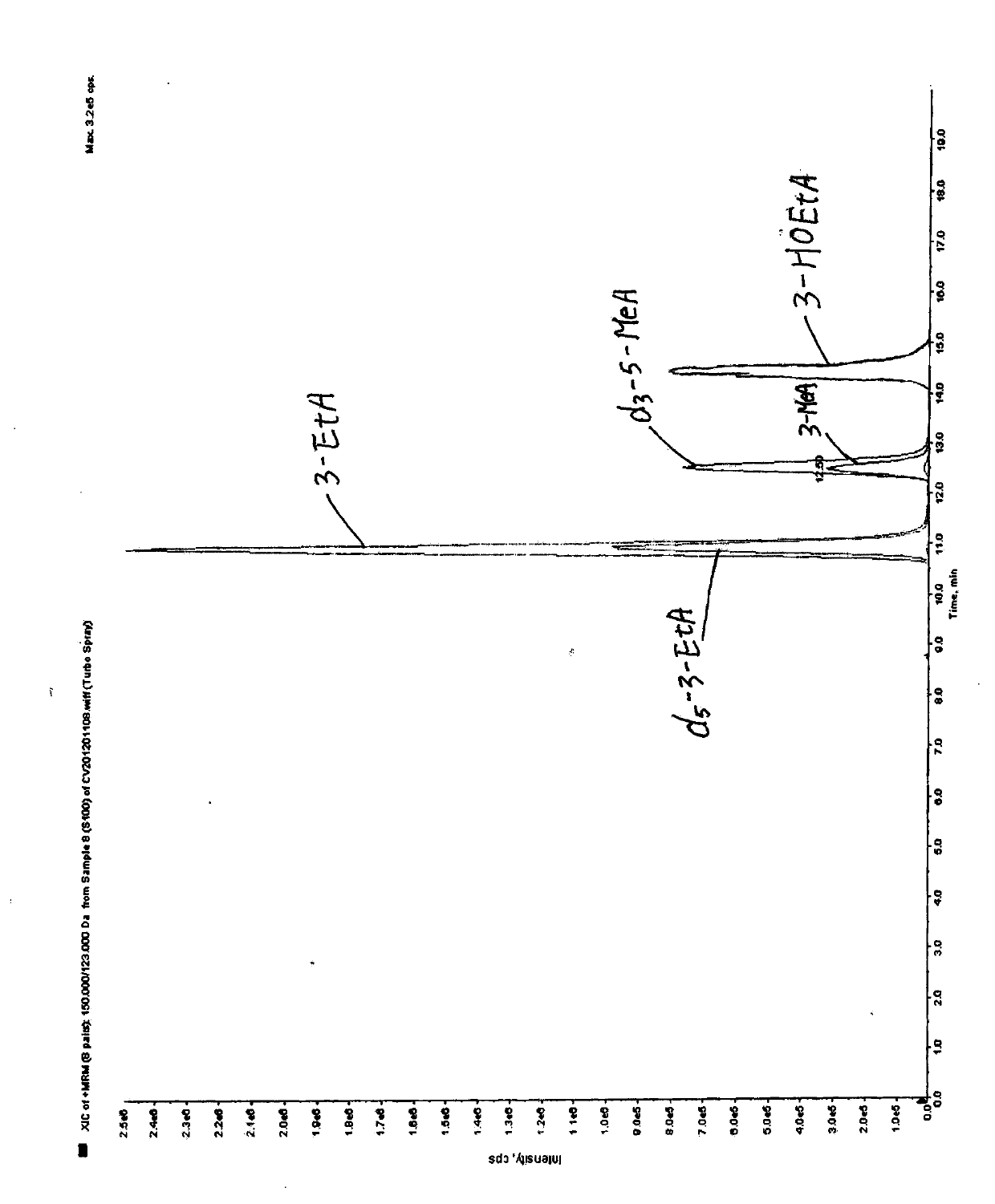
[0055] Agilent 1200 liquid chromatography (Agilent, USA), equipped with G1367D autosampler, G 1312B binary mixing pump and G1316B column thermostat; API 5500 triple quadrupole tandem mass spectrometer (Applied Biosystems, USA), equipped with Electrospray ion source (ESI) and Analyst 1.5 software data processing system.
[0056] Ammonia was analytically pure; ammonium formate, ethyl acetate, and methanol were all chromatographically pure (TEDIA, USA); Oasis MCX solid-phase extraction column (6 mL, Waters, USA).
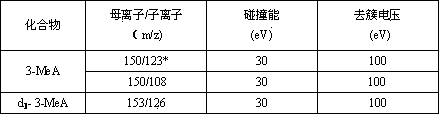
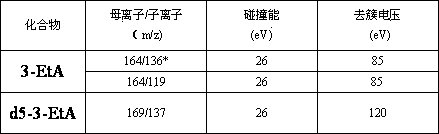
[0057] 3-methyladenine, 3-ethyladenine, and 3-hydroxyethyladenine were purchased from Canada TRC Company, deuterated-3-methyladenine, deuterated-3-ethyladenine internal standard was purchased from American CIL laboratory.
[0058] 2. Sample handling:
[0059] The urine was thawed at room temperature and mixed evenly. Take 4 mL of urine, add 4 mL of ultrapure water, add 100 μL of internal standard (100 ng / mL), and adjust the tem...
example 2
[0061] Example 2: As described in Example 1, the method established in this study was used to detect the content of 3-alkylated adenine in the urine of 20 samples. The content of 3-alkylated adenine in the urine is shown in Table 6.
[0062] Table 6 Content of 3-alkylated adenine in urine
[0063] serial number 3-MeA content / 24h (μg) 3-EtA content / 24h (μg) 3-HOEtA content / 24h (μg) serial number 3-MeA content / 24h (μg) 3-EtA content / 24h (μg) 3-HOEtA content / 24h (μg) 1 5.72 108.4 228.8 11 25.64 955.4 120.7 2 13.16 417.4 364.2 12 16.48 142.9 295.9 3 21.75 313.0 345.6 13 25.88 781.6 106.2 4 10.12 237.3 211.5 14 28.45 939.3 332.9 5 6.73 546.7 726.1 15 26.87 399.5 323.2 6 27.73 277.3 144.7 16 28.45 636.0 153.3 7 63.14 216.6 696.8 17 24.58 494.8 154.0 8 12.87 245.3 281.7 18 69.58 572.5 747.0 9 77.35 246.7 172.6 19 29.38 220.7 185.7 10 45.06 301.8 282.3 20 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com