Application of hyaluronic acid combined with lauromacrogol in preparation of foam sclerosing medicine for treating venous malformation
A technology of hyaluronic acid and venous malformation, which can be applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problem of limited effect of venous malformation treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
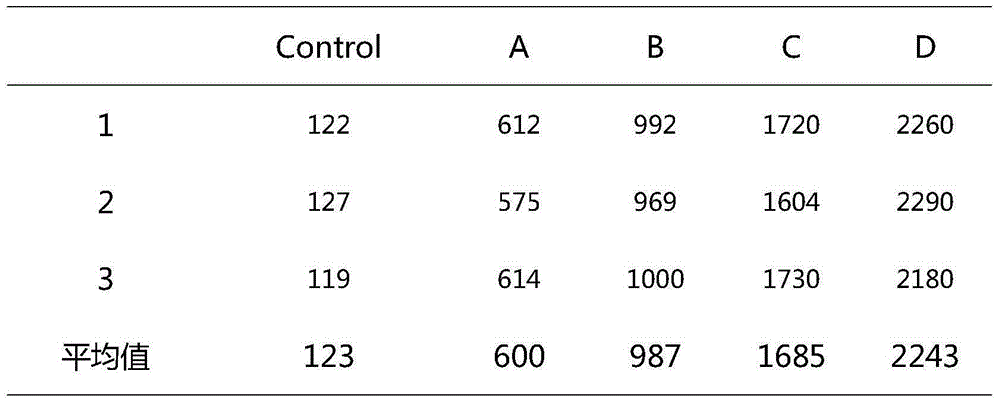
[0021] Embodiment 1 Hyaluronic acid is combined with lauromacrogol to make a foam hardener and apply it
[0022] The preparation method of foam hardening agent is as follows:
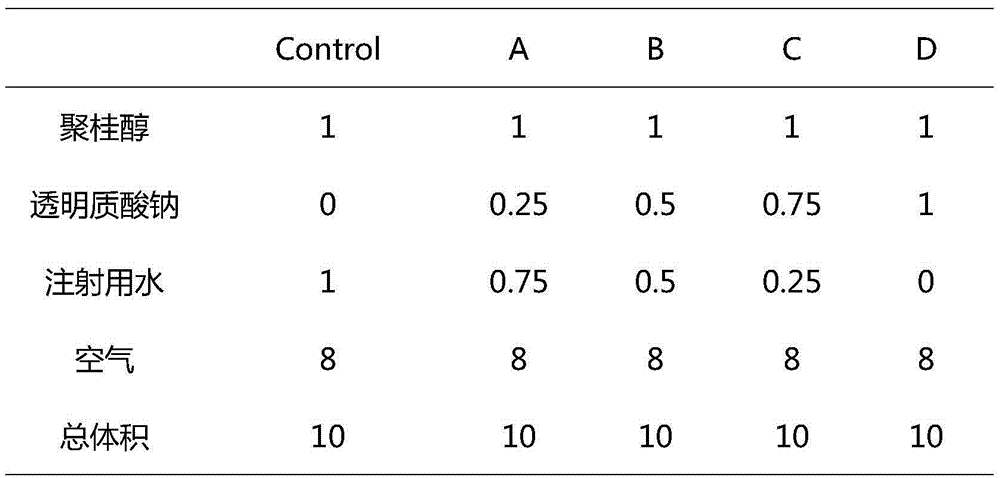
[0023] Use one syringe to extract lauromacrogol, hyaluronic acid and water for injection, shake to mix evenly, use another syringe to extract sterile air, connect the two syringes through a medical three-way valve, follow the Tessari method (the most classic method for preparing foam sclerosing agents) , the most commonly used method) several times before and after bolus mixing to form a uniform and stable foam; among them, the volume ratios of lauromacrogol, hyaluronic acid, water for injection, and sterile air are shown in Table 1 (four experimental groups, a control group).
[0024] Table 1 Experimental grouping
[0025]
[0026] Note:
[0027] Volume unit mL
[0028] Lauromacrogol used in the present invention is commercially available, chemical name: polyoxyethylene lauryl ether, Shaanxi Tia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com