Copolymer containing cyclopentadiene bithiophene-benzo-bis (benzothiadiazole) and preparation method and application thereof
A dithiazole, copolymer-like technology, applied in chemical instruments and methods, semiconductor/solid-state device manufacturing, organic chemistry, etc., can solve problems such as energy gap width
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
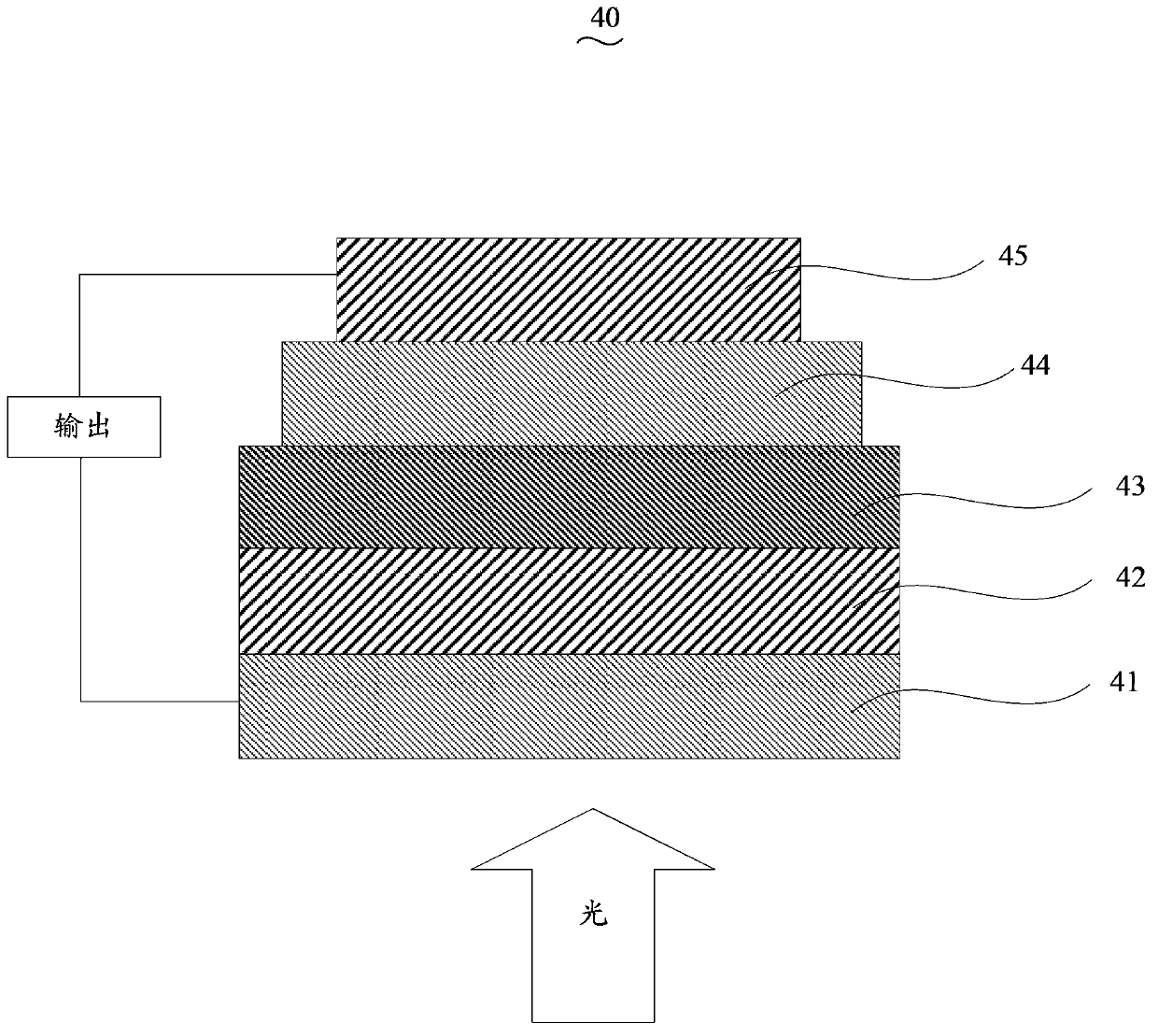
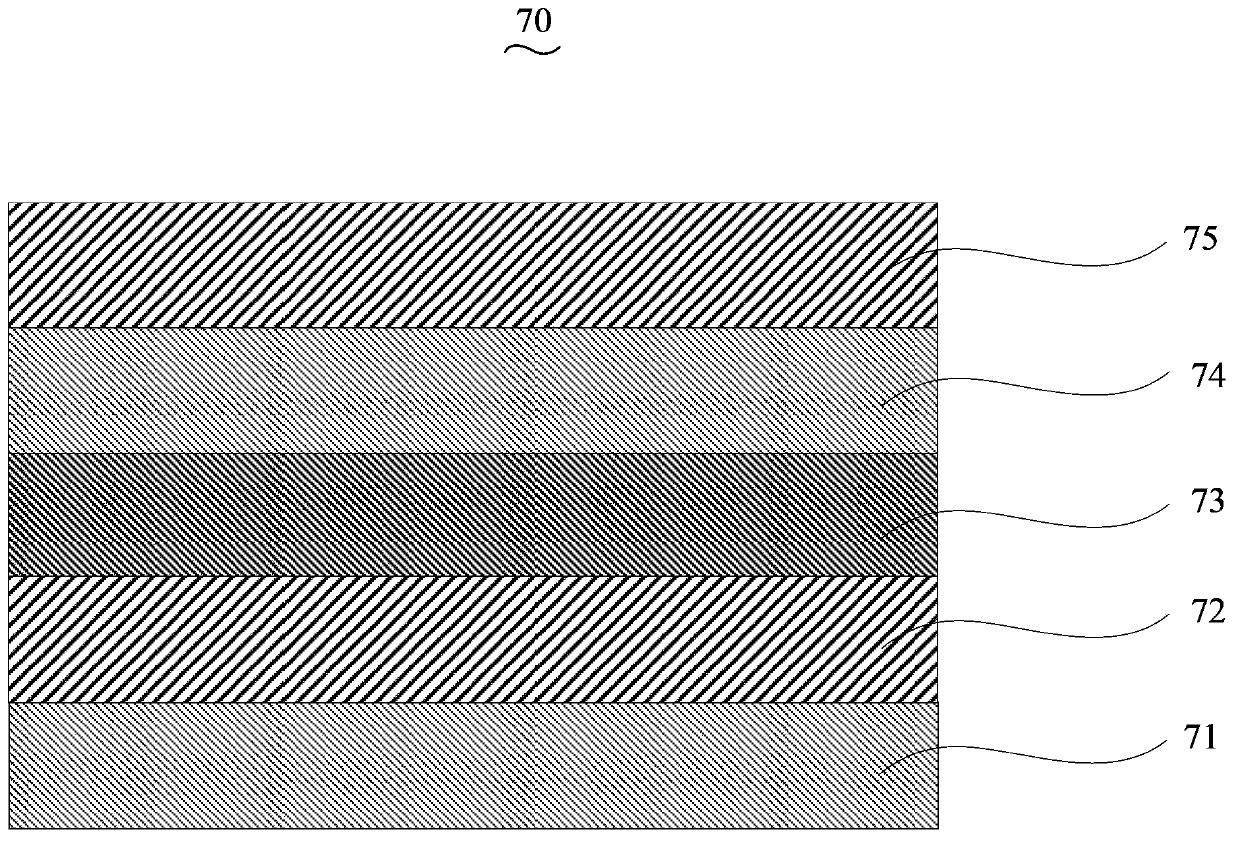
Image
Examples
preparation example Construction
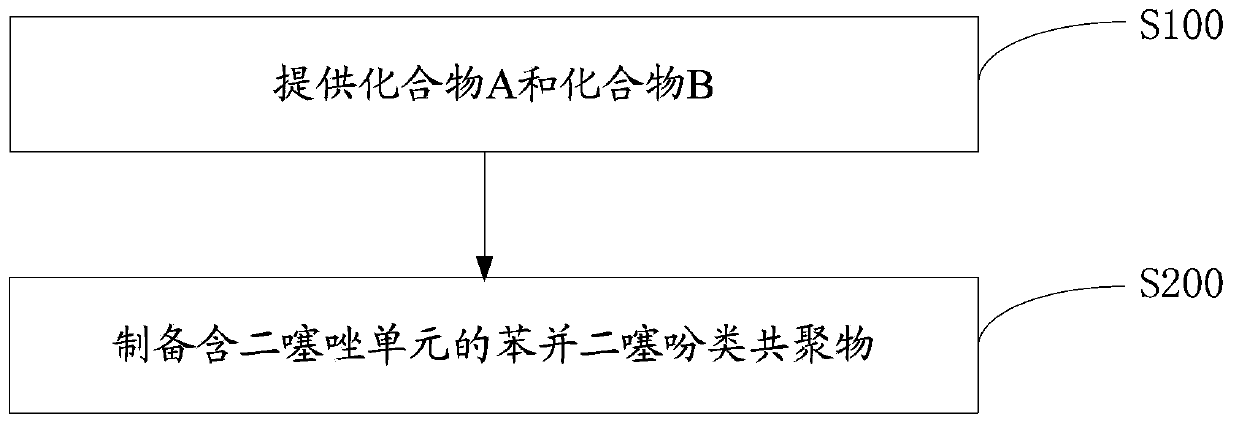
[0058] The preparation method of the benzodithiophene copolymer containing bithiazole unit of one embodiment, such as figure 1 shown, including the following steps:
[0059] Step S100, providing compound A and compound B.
[0060] A is: B is:
[0061] Among them, R 1 , R 2 , R 5 , R 6 for H, C 1 ~C 16 the alkyl group, R 3 for C 1 ~C 16 Alkyl, C 1 ~C 16 The alkoxy group or the alkylthiophene represented by the following structural formula:
[0062] R is C 1 ~C 16 the alkyl group, R 4 for H, C 1 ~C 16 Alkyl, C 1 ~C 16 of alkoxy.
[0063] Wherein the preparation of compound A comprises the following steps:
[0064] Step S111, under the protection of nitrogen, add the dichloromethane solution of compound C represented by the following structural formula dropwise to the dichloromethane solution containing 1,3-dicyclohexylcarbodiimide (DDC) and 4-dimethylaminopyridine (DMAP) Reaction in methyl chloride at room temperature for 12 hours to 15 hours, wherein...
Embodiment 1
[0103] This embodiment discloses a benzodithiophene copolymer with the following structure:
[0104]
[0105] n=60;
[0106] The preparation steps of above-mentioned benzodithiophene copolymer are as follows:
[0107] One, the preparation of 1,3-two (2-thiophene) acetone:
[0108]
[0109] First, dissolve 7.6g (36.8mmol) of DCC and 1.23g (10mmol) of DMAP with 70mL of treated dichloromethane, and dissolve 5g (35.2mmol) of 2-thiopheneacetic acid in 70mL of dichloromethane under the protection of nitrogen Add dropwise to the above reaction solution, and react for 12 hours. After the reaction, the reaction solution was filtered, recrystallized twice with n-hexane, and then separated and purified by column chromatography to obtain the product.
[0110] MALDI-TOF-MS (m / z): 222.3 (M + ).
[0111] Two, the preparation of 2,7-dioctyloxybenzo[1,2-b:4,3-b']dithiophene-4,5-dione:
[0112]
[0113] Take 25.4g (60mmol) of 4,4'-bis(2-octyloxy)thiophene and add it to 400mL of d...
Embodiment 2
[0128] This embodiment discloses a benzodithiophene copolymer with the following structure:
[0129]
[0130] n=30;
[0131] The preparation steps of above-mentioned benzodithiophene copolymer are as follows:
[0132] One, the preparation of 1,3-two (3-methyl-2-thiophene) acetone:
[0133]
[0134] First, dissolve 8.3g (40mmol) of DCC and 1.23g (10mmol) of DMAP with 70mL of treated dichloromethane, and dissolve 5g (35.2mmol) of 3-methyl-2-thiopheneacetic acid in 70mL of The dichloromethane solution was added dropwise to the above reaction solution, and reacted for 15 hours. After the reaction, the reaction solution was filtered, recrystallized twice with n-hexane, and then separated and purified by column chromatography to obtain the product.
[0135] MALDI-TOF-MS (m / z): 250 (M + ).
[0136] 2. Preparation of 2,7-di(hexadecyloxy)benzo[1,2-b:4,3-b']dithiophene-4,5-dione:
[0137]
[0138] Take 33.5g (51.8mmol) of 4,4'-bis(2-hexadecyloxy)thiophene and add it to 40...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com