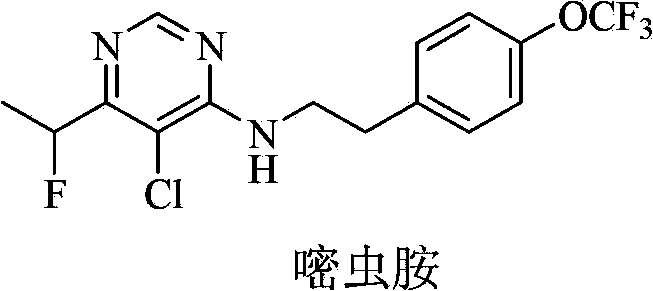
Fluorine-containing pyrimidine compound and application
A technology of compounds and pyrimidines, which is applied in the field of fluoropyrimidine compounds, and can solve the problems that the structure of fluoropyrimidine compounds has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0370] Embodiment 1: the preparation of intermediate 4,5-dichloro-6-difluoromethylpyrimidine
[0371] 1) Preparation of ethyl 2-chloro-4,4-difluoroacetoacetate
[0372]
[0373] Slowly add 177.46 g (1.33 mol) of sulfuryl chloride in 200 ml of dichloromethane solution dropwise to 200.00 g (1.20 mol) of ethyl 4,4-difluoroacetoacetate in 300 ml of dichloromethane under stirring at room temperature, and add for about 3 hours. After completion, a large amount of gas was generated, and the stirring reaction at room temperature was continued for 5-7 hours. After the reaction was monitored by TLC, the dichloromethane solvent and excess sulfuryl chloride were evaporated under reduced pressure to obtain 240 g of a light yellow liquid.
[0374] 2) Preparation of 4-hydroxy-5-chloro-6-difluoromethylpyrimidine
[0375]
[0376] Take 71.9g (0.70mol) of formamidine acetate in a 1000ml there-necked flask, add 150ml of methanol, stir at 5-10°C, take 64.6g (120mol) of sodium methoxide, co...
Embodiment 2
[0380] Embodiment 2: Preparation of intermediate 2-(4-(5-(trifluoromethyl)pyridine-2-oxyl)phenyl)ethylamine
[0381] 1) Preparation of 2-(4-(5-(trifluoromethyl)pyridine-2-oxyl)phenyl)acetonitrile
[0382]
[0383] Add 18.15g (0.1mol) of 2-chloro-5-trifluoromethylpyridine and 15.96g (0.12mol) of p-hydroxybenzonitrile into 200ml of butanone, add 27.60g (0.2mol) of potassium carbonate, and heat to reflux under stirring , reacted for 4-10 hours, after the reaction was monitored by TLC, the solvent was evaporated under reduced pressure, and 300ml of ethyl acetate was added for extraction. Column chromatography (eluent: ethyl acetate and petroleum ether (boiling range 60-90°C), volume ratio: 1:4) gave 22.50 g of white solid, yield 81%, melting point 48-49°C.
[0384] 2) Preparation of 2-(4-(5-(trifluoromethyl)pyridine-2-oxyl)phenyl)ethylamine
[0385]
[0386] A mixture of 2.78g (0.01mol) 2-(4-(5-(trifluoromethyl)pyridine-2-oxyl)phenyl)acetonitrile, Raney nickel (1.0g), 25% ...
Embodiment 3
[0387] Example 3: Preparation of intermediate 2-(4-(3,5,6-tri(chloro)pyridine-2-oxyl)phenyl)ethylamine hydrochloride
[0388] 1) Preparation of N-Boc-4-hydroxyphenethylamine
[0389]
[0390] Dissolve 11.3g (0.1mol) of 4-hydroxyphenethylamine in 80ml of tetrahydrofuran, add 10.08g (0.12mol) of sodium bicarbonate and 50ml of water in turn, and add 21.80g (0.1mol) of di-tert-butyl dicarbonate dropwise under stirring at room temperature After dropping the ester, continue to react for 4-10 hours. After the reaction is monitored by TLC, the solvent is evaporated under reduced pressure, and (3×50ml) ethyl acetate is added for extraction. Column chromatography (eluent is ethyl acetate and petroleum ether (boiling range 60-90°C), volume ratio: 1:4) gave 17.15 g of white solid, yield 81%, melting point 48-49°C.
[0391] 2) Preparation of N-Boc-2-(4-(3,5,6-trichloropyridine-2-oxyl)phenyl)ethylamine
[0392]
[0393] Add 2.37g (0.01mol) N-Boc-4-hydroxyphenethylamine and 2.17g (0....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com