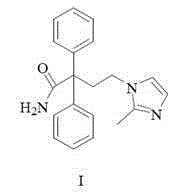
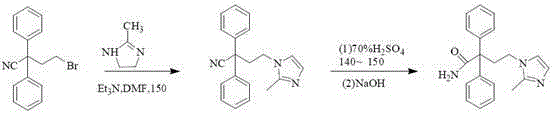
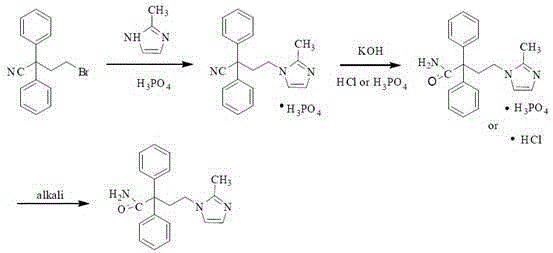
A kind of method for preparing midanaxin
A midanaxine and route technology, applied in the field of preparation of midanaxine, can solve the problems of low safety, unfriendly human body and environment, blindness, etc., and achieve the effect of being friendly to human body and environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation of 4-bromo-2,2-diphenylbutyronitrile
[0030] Add 400mL dry tetrahydrofuran to a 2L reaction flask, add 60% sodium hydride (82.8g, 2.06mol) under nitrogen protection, and stir to obtain gray cloudy liquid A. Fully dissolve diphenylacetonitrile (200g, 1.04mol) and 1,2-dibromoethane (204.2g, 1.08mol) with 400mL dry tetrahydrofuran solution to obtain a colorless clear liquid B; Drop B into the turbid solution A, and keep it warm at 10-15°C for 6 hours. After the reaction is detected by TLC, add a small amount of water dropwise to the reaction system until no bubbles are generated. Then add 800mL of water and 400mL of ethyl acetate, stir, separate the layers, wash the organic layer with water and saturated sodium chloride solution, dry the organic layer with anhydrous sodium sulfate, filter with suction, and concentrate under reduced pressure to obtain 310g of yellow liquid.
[0031] The obtained yellow liquid was stirred and dissolved with 800 mL...
Embodiment 2
[0032] Embodiment 2: Preparation of 4-bromo-2,2-diphenylbutanamide
[0033] Add 4-bromo-2,2-diphenylbutyronitrile (150g, 0.5mol), 750mL 6mol / L KOH solution, 750mL dimethyl sulfoxide into a 3L reaction flask, heat to 100-120°C and stir for reaction for 1 hour. After the TLC detection reaction is completed, cool down to 40°C, add 2000mL of water and 2000mL of dichloromethane, stir, separate the layers, wash the organic layer with water, saturated sodium bicarbonate and sodium chloride solution, separate the layers, and dry the organic layer with anhydrous sodium sulfate , suction filtration, and concentration under reduced pressure to obtain 161.92 g of brown oily liquid, with a yield of 96%.
Embodiment 3
[0034] Embodiment 3: Preparation of 4-bromo-2,2-diphenylbutanamide
[0035] Add 4-bromo-2,2-diphenylbutyronitrile (150g, 0.5mol), 666mL6mol / L NaOH solution, 750mL dimethyl sulfoxide into a 3L reaction flask, heat to 100-120°C and stir for reaction for 1h. After the TLC detection reaction is completed, cool down to 40°C, add 2000mL of water and 2000mL of dichloromethane, stir, separate the layers, wash the organic layer with water, saturated sodium bicarbonate and sodium chloride solution, separate the layers, and dry the organic layer with anhydrous sodium sulfate , suction filtration, and concentration under reduced pressure to obtain 146.73 g of brown oily liquid with a yield of 87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com