A kind of preparation method of erythromycin thiocyanate
A technology of erythromycin thiocyanate and erythromycin, which is applied in the directions of sugar derivatives and organic chemistry, can solve the problems of many impurities and low purity, and achieve the effects of high purity, excellent selectivity and low boiling point.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
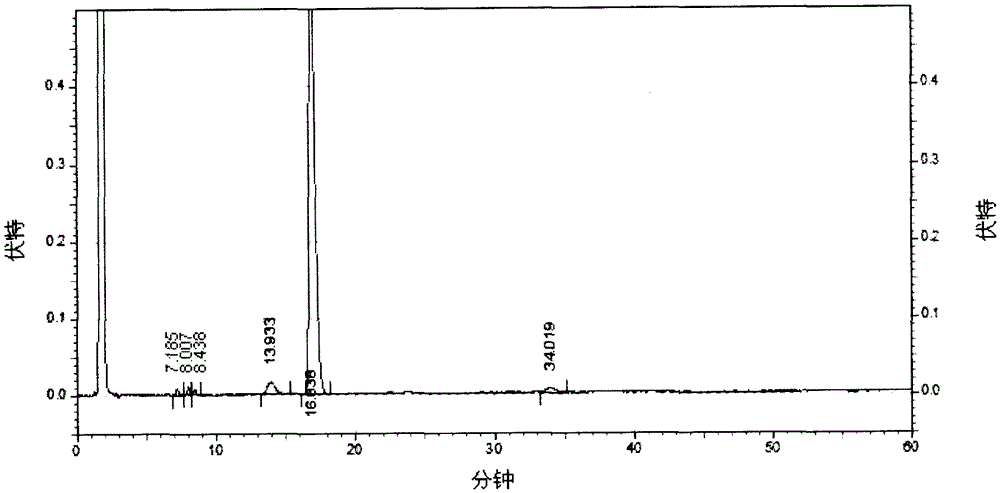
[0047] Take 100g of crude erythromycin, add 600mL of acetone, place in a water bath and heat to 35°C, adjust the pH to 8.5, filter to obtain the filtrate, keep the filtrate at 35°C, add sodium thiocyanate, the molar ratio is 2:1, After stirring to make it dissolve, use acetic acid to adjust the pH of the solution to 6.0, slowly cool down to 10°C, keep warm for about 15 minutes, then centrifuge, rinse with 100mL purified water, crush and dry to obtain erythromycin thiocyanate, the main component of which is red The content of mycin A is 85.1% (detected by HPLC method, calculated as dry product).
Embodiment 2
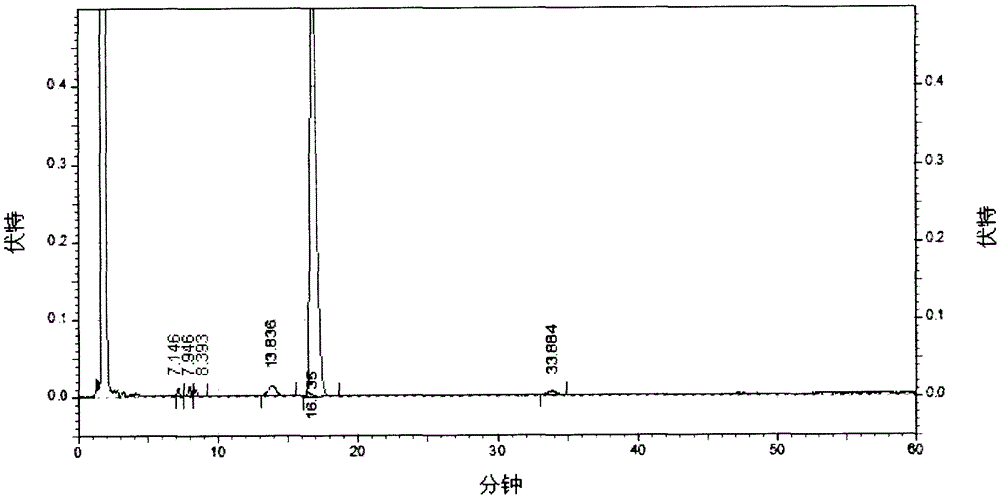
[0049] Take 150g of crude erythromycin thiocyanate, add 600mL of acetone, place in a water bath and heat to 45°C, adjust the pH to 10.0, filter to obtain the filtrate, keep the filtrate at 45°C, add potassium thiocyanate, the molar ratio of addition is 0.2 : 1, stir to make it dissolve, use dilute hydrochloric acid to adjust the pH of the solution to 7.5, slowly cool down to 0°C, keep warm for about 15 minutes, centrifuge, use 100mL purified water to rinse, pulverize, and dry to obtain erythromycin thiocyanate. Its main component, erythromycin A, has a content of 84.7% (detected by HPLC method, calculated as dry product).
Embodiment 3
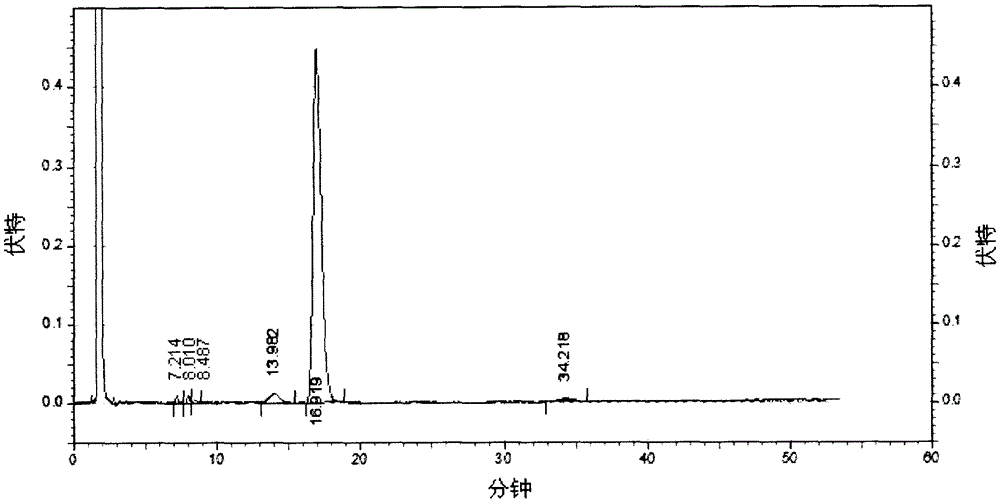
[0051] Take 150g of crude erythromycin lactate, add 600mL of acetone, place in a water bath and heat to 40°C, adjust the pH to 9.0, filter to obtain the filtrate, keep the filtrate at 45°C, add ammonium thiocyanate, the molar ratio of addition is 2:1 , stir to make it dissolve, use dilute hydrochloric acid to adjust the pH of the solution to 7.5, slowly cool down to 5°C, keep it warm for about 15 minutes, then centrifuge, rinse with 100mL of purified water, pulverize and dry to obtain erythromycin thiocyanate. The content of component erythromycin A is 84.9% (detected by HPLC method, calculated as dry product).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com