Preparation method of multi-morphologynano-sized zinc ferrite
A technology of nano-zinc ferrite and zinc ferrite, which is applied in the direction of nanotechnology, chemical instruments and methods, iron compounds, etc., can solve the problems of ineffective control of shape changes, inconvenient operation, limiting industrial application and production efficiency, etc. Achieve the effect of broad application market and prospect, improve production efficiency and reduce production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Weigh 2.2g of zinc acetate dihydrate, 8.08g of ferric nitrate nonahydrate and 13g of polyvinyl alcohol (model 1750±50, Sinopharm Chemical Reagent Co., Ltd.), add them to 105ml of deionized water, stir well, and obtain a mixed solution;
[0031] The mixed solution was electrostatically sprayed to obtain a precursor with a spherical shape, wherein the electrostatic voltage was 10 kV, and the spraying distance was 8 cm;
[0032] The precursor with spherical shape was heated up to 350°C at a pre-calcination rate of 5°C / min and kept for 2 hours, and then heated to 1000°C with a calcination rate of 5°C / min and kept for 1 hour, and cooled naturally to obtain material 1 .
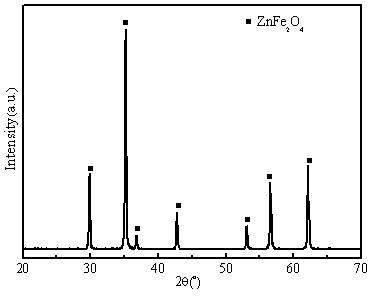
[0033] Adopt X-ray diffraction analyzer (XRD, Model X'TRAX) to carry out phase composition analysis, the result is as follows figure 1 As shown, it can be seen that material 1 is pure phase zinc ferrite (ZnFe 2 o 4 ). A cold field emission scanning electron microscope (FESEM, S-4800) was used t...
Embodiment 2
[0035] Weigh 1.36g of zinc chloride, 7.06g of iron acetylacetonate and 18g of polyvinylpyrrolidone K-30 (Sinopharm Chemical Reagent Co., Ltd.) into 100ml of ethanol solution, stir well to obtain a mixed solution;
[0036] The mixed solution was electrostatically sprayed to obtain a precursor with a fibrous shape, wherein the electrostatic voltage was 15 kV, and the spraying distance was 13 cm;
[0037] The precursor of fibrous morphology was raised to 500°C at a pre-calcination heating rate of 1°C / min and kept for 4 hours, then raised to 700°C at a calcination heating rate of 1°C / min and held for 5 hours, and naturally cooled to obtain the material 2 4 .
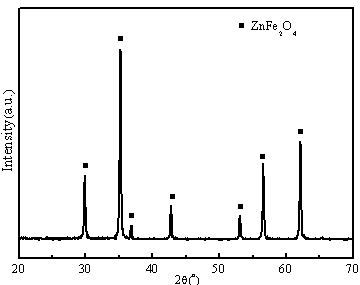
[0038] Adopt X-ray diffraction analyzer (XRD, Model X'TRAX) to carry out phase composition analysis, the result is as follows image 3 As shown, it can be seen that material 2 is pure phase zinc ferrite (ZnFe 2 o 4 ). A cold field emission scanning electron microscope (FESEM, S-4800) was used to test the micro...
Embodiment 3
[0040] Weigh 2.2 g of zinc acetate dihydrate, 5.41 g of ferric chloride hexahydrate and 10 g of polyethylene oxide (molecular weight 1,000,000, Shanghai Liansheng Chemical Co., Ltd.), add them to 35 ml of N,N-dimethylformamide solution, stir well, get a mixed solution;
[0041] The mixed solution was electrostatically sprayed to obtain a precursor with a bead-shaped morphology, wherein the electrostatic voltage was 20 kV, and the spraying distance was 20 cm;
[0042] The rod-shaped precursor was heated up to 400°C at a pre-calcination rate of 3°C / min and kept for 3 hours, then heated to 900°C at a calcination rate of 3°C / min and kept for 3 hours, and cooled naturally to obtain material 3 .
[0043] Adopt X-ray diffraction analyzer (XRD, Model X'TRAX) to carry out phase composition analysis, the result is as follows Figure 5 As shown, it can be seen that material 3 is pure phase zinc ferrite (ZnFe 2 o 4 ). A cold field emission scanning electron microscope (FESEM, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com